Abstract
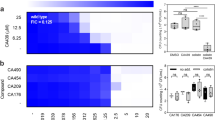
The emergence of multidrug-resistant pathogens, particularly β-lactam, colistin, and fosfomycin-resistant Escherichia coli and Salmonella, is a significant public health concern. This study evaluated the in vitro synergistic effects of antimicrobial combinations against these resistant isolates. Ten isolates that originated from retail chicken meat, including five E. coli and five Salmonella isolates, were tested against cefotaxime (CTA), fosfomycin (FOS), and colistin (COL), both individually and in combinations. Antimicrobial susceptibility was assessed using the broth microdilution method, and synergistic interactions were evaluated using checkerboard and time-killing assays. All isolates were multidrug-resistant (MDR) and were resistant to CTA, COL, and FOS. The checkerboard assay showed varying levels of synergy: two out of five E. coli isolates exhibited synergy with FOS-COL, while one E. coli isolates out of four isolates showed synergy with CTA-COL. No E. coli isolates showed synergy with FOS-CTA. For Salmonella, two out of five isolates exhibited synergy with both FOS-CTA and FOS-COL, while three out of four isolates showed synergy with CTA-COL. The time-killing assay confirmed these results, with the FOS-COL combinations showing synergy against both E. coli and Salmonella strains. Notably, the FOS-COL combination demonstrated bactericidal effects against E. coli, and all three combinations were bactericidal against Salmonella. The study highlights the potential of antimicrobial combinations, particularly FOS-COL, in combating MDR E. coli and Salmonella. These findings support the use of combination therapy as a promising strategy to in effectively treating multi-drug-resistant foodborne infections, ensuring better medical outcomes and enhanced food safety, warranting further investigation into their mechanisms and clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Khalifa HO, Okanda T, Abd El-Hafeez AA, Abd El Latif A, Habib AG, Yano H, et al. Comparative evaluation of five assays for detection of carbapenemases with a proposed scheme for their precise application. J Mol Diagn. 2020;22:1129–38.
Khalifa HO, Soliman AM, Saito T, Kayama S, Yu L, Hisatsune J, et al. First report of foodborne Klebsiella pneumoniae coharboring blaVIM-1, blaNDM-1, and mcr-9. Antimicrob Agents Chemother. 2020;64:e00882-20.
Khalifa HO, Oreiby AF, Abd El-Hafeez AA, Okanda T, Haque A, Anwar KS, et al. First report of multidrug-resistant carbapenemase-producing bacteria coharboring mcr-9 associated with respiratory disease complex in pets: Potential of animal-human transmission. Antimicrob Agents Chemother. 2021;65:e01890-20.
de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13:e1002184.
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–55.
European Civil Protection and Humanitarian Aid Operations. Global food crisis: what you need to know in 2023. Available at: https://civil-protection-humanitarian-aid.ec.europa.eu/news-stories/stories/global-food-crisis-what-you-need-know-2023_en.
Coulombier D, Takkinen J. From national to international—challenges in cross-border multi-country, multi-vehicle foodborne outbreak investigations. Euro Surveill. 2013;18:20423.
Iturriza-Gomara M, O’Brien SJ. Foodborne viral infections. Curr Opin Infect Dis. 2016;29:495–501.
Nyachuba DG. Foodborne illness: is it on the rise? Nutr Rev. 2010;68:257–69.
Ke BX, He DM, Tan HL, Zeng HH, Yang T, Li BS, et al. Active etiological surveillance for foodborne diseases in Guangdong province, 2013-2014. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:1373–8.
Okyere A, Bishoff D, Oyaro MO, Ajami NJ, Darkoh C. Analysis of fish commonly sold in local supermarkets reveals the presence of pathogenic and multidrug-resistant bacterial communities. Microbiol Insights. 2018;11:1178636118786925.
Page AV, Liles WC. Enterohemorrhagic Escherichia coli infections and the hemolytic-uremic syndrome. Med Clin North Am. 2013;97:681–95.
Ma Y, Ding S, Fei Y, Liu G, Jang H, Fang J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control. 2019;106:106712.
Hoffmann S, Ahn JW. Economic cost of major foodborne illnesses increased $2 billion from 2013 to 2018. Amber Waves. 2021;2021:04.
World Health Organization. Critically important antimicrobials for human medicine, 6th rev. World Health Organization, Geneva, Switzerland. 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf.
Cameron-Veas K, Solà-Ginés M, Moreno MA, Fraile L, Migura-Garcia L. Impact of the use of β-Lactam antimicrobials on the emergence of Escherichia coli isolates resistant to cephalosporins under standard pig-rearing conditions. Appl Environ Microbiol. 2015;81:1782–7. https://doi.org/10.1128/aem.03916-14.
Khalifa HO, Oreiby A, Abd El-Hafeez AA, Abd El Latif A, Okanda T, Kato Y, et al. High β-Lactam and quinolone resistance of Enterobacteriaceae from the respiratory tract of sheep and goat with respiratory disease. Animals. 2021;11:2258 https://doi.org/10.3390/ani11082258.
Khalifa HO, Oreiby AF, Okanda T, Kato Y, Matsumoto T. High β-lactam resistance in Gram-negative bacteria associated with kennel cough and cat flu in Egypt. Sci Rep. 2021;11:82061 https://doi.org/10.1038/s41598-021-82061-2.
Habib I, Elbediwi M, Mohamed MYI, Ghazawi A, Abdalla A, Khalifa HO, et al. Enumeration, antimicrobial resistance and genomic characterization of extended-spectrum β-lactamases producing Escherichia coli from supermarket chicken meat in the United Arab Emirates. Int J Food Microbiol. 2023;398:110224.
Khalifa HO, Ahmed AM, Oreiby AF, Eid AM, Shimamoto T, Shimamoto T. Characterisation of the plasmid-mediated colistin resistance gene mcr-1 in Escherichia coli isolated from animals in Egypt. Int J Antimicrob Agents. 2016;47:413–4.
Habib I, Elbediwi M, Mohteshamuddin K, Mohamed MYI, Lakshmi GB, Abdalla A, et al. Genomic profiling of extended-spectrum β-lactamase-producing Escherichia coli from pets in the United Arab Emirates: Unveiling colistin resistance mediated by mcr-1.1 and its probable transmission from chicken meat – A One Health perspective. J Infect Public Health. 2023;16:163–71.
Khalifa HO, Shikoray L, Mohamed MYI, Habib I, Matsumoto T. Veterinary drug residues in the food chain as an emerging public health threat: Sources, analytical methods, health impacts, and preventive measures. Foods. 2024;13:1629 https://doi.org/10.3390/foods13111629.
Habib I, Elbediwi M, Ghazawi A, Mohamed MY, Lakshmi GB, Khan M. First report from supermarket chicken meat and genomic characterization of colistin resistance mediated by mcr-1.1 in ESBL-producing, multidrug-resistant Salmonella Minnesota. Int J Food Microbiol 2022;379:109835.
Habib I, Mohamed MY, Lakshmi GB, Ghazawi A, Khan M, Abdalla A, et al. High prevalence and genomic features of multidrug-resistant Salmonella enterica isolated from chilled broiler chicken on retail sale in the United Arab Emirates. Int J Food Microbiol 2024;423:110828.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 32nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, M100, 34th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2024.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-second informational supplement update. CLSI document M100-S22 U. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
Bellio P, Fagnani L, Nazzicone L, Celenza G. New and simplified method for drug combination studies by checkerboard assay. MethodsX. 2021;8:101543.
Odds FC. Synergistic, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1.
Khalifa HO, Majima H, Watanabe A, Kamei K. In vitro characterization of twenty-one antifungal combinations against echinocandin-resistant and -susceptible Candida glabrata. J Fungi. 2021;7:108.
Eliopoulos GM, Moellering RC Antimicrobial combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine. 4th ed. Baltimore, MD: The Williams and Wilkins Co; 1996. p. 330–96.
Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25:450–70.
Muteeb G, Rehman MT, Shahwan M, Aatif M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals. 2023;16:1615.
Murugaiyan J, Kumar PA, Rao GS, Iskandar K, Hawser S, Hays JP, et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics. 2022;11:200.
Albur MS, Noel A, Bowker K, MacGowan A. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int J Antimicrob Agents. 2015;46:560–7.
Wang J, He JT, Bai Y, Wang R, Cai Y. Synergistic activity of colistin/fosfomycin combination against carbapenemase‐producing Klebsiella pneumoniae in an in vitro pharmacokinetic/pharmacodynamic model. Biomed Res Int. 2018;2018:5720417.
Saelim W, Changpradub D, Thunyaharn S, Juntanawiwat P, Nulsopapon P, Santimaleeworagun W. Colistin plus sulbactam or fosfomycin against carbapenem-resistant Acinetobacter baumannii: improved efficacy or decreased risk of nephrotoxicity? Infect Chemother. 2021;53:128.
Di X, Wang R, Liu B, Zhang X, Ni W, Wang J, et al. In vitro activity of fosfomycin in combination with colistin against clinical isolates of carbapenem-resistant Pseudomonas aeruginosa. J Antibiot (Tokyo). 2015;68:551–5.
Ontong JC, Ozioma NF, Voravuthikunchai SP, Chusri S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS One. 2021;16:e0244673.
Al Atya AK, Drider-Hadiouche K, Vachee A, Drider D. Potentialization of β-lactams with colistin: In case of extended spectrum β-lactamase producing Escherichia coli strains isolated from children with urinary infections. Res Microbiol. 2016;167:215–21.
Karaoglan I, Zer Y, Bosnak VK, Mete AO, Namiduru M. In vitro synergistic activity of colistin with tigecycline or β-lactam antibiotic/β-lactamase inhibitor combinations against carbapenem-resistant Acinetobacter baumannii. J Int Med Res. 2013;41:1830–7.
Gouyon JB, Duez JM, Portier H, Brichon P, Kohli E, Alison M. Fosfomycin-cefotaxime combination in severe staphylococcal infections in newborn infants. Presse Med. 1985;14:2135–8.
Pestel M, Martin E, Aucouturier C, Lemeland JF, Caron F. In vitro interactions between different beta-lactam antibiotics and fosfomycin against bloodstream isolates of enterococci. Antimicrob Agents Chemother. 1995;39:2341–5.
Ks S, Pallam G, Mandal J, Jindal B. Use of fosfomycin combination therapy to treat multidrug-resistant urinary tract infection among pediatric surgical patients–a tertiary care centre experience. Access Microbiol. 2020;2:e000163.
Windels EM, Van den Bergh B, Michiels J. Bacteria under antibiotic attack: different strategies for evolutionary adaptation. PLoS Pathog. 2020;16:e1008431.
Sanabria J, Garzón V, Pacheco T, Avila M-P, Garcia J-C, Jaimes D, et al. Estimation of the difference in colistin plasma levels in critically Ill patients with favorable or unfavorable clinical outcomes. Pharmaceutics. 2021;13:1630 https://doi.org/10.3390/pharmaceutics13101630.
Binsker U, Käsbohrer A, Hammerl JA. Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol Rev 2022;46:fuab049.
Funding
This research was supported by the United Arab Emirates University (UAEU) Start-up (proposal number 3219) and Sure Plus (proposal number 2837) grants for H.O.K. M.-Y.I.M is a post-doc fellow funded by ASPIRE, the technology program management pillar of Abu Dhabi’s Advanced Technology Research Council (ATRC), via the ASPIRE Research Institute for Food Security in the Drylands (ARIFSID) project (Subtheme 4.1—One Health and Antimicrobial Resistance).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalifa, H.O., Mohammed, T., Mohamed, MY.I. et al. In vitro assessment of the synergistic effects of cefotaxime, colistin, and fosfomycin combinations against foodborne resistant Escherichia coli and Salmonella isolates. J Antibiot 78, 265–273 (2025). https://doi.org/10.1038/s41429-025-00808-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-025-00808-9