Abstract
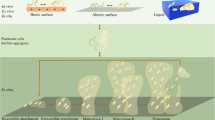
In this study, three KRN7000 analogues with variations in the sugar and glycosidic linkage were synthesised to assess their efficacy in disrupting the biofilms of S. pyogenes and P. mirabilis. All three analogues exhibited antibacterial activity, with the effects being more prominent at lower concentrations in S. pyogenes. The N-alkylated, 1-deoxy analogue emerged as the most effective, significantly reducing biofilm formation and extracellular polymeric substances (EPS) in both organisms. Microscopic analysis revealed notable disruption of biofilm structure by the analogue, resulting in a significant reduction in EPS for both organisms and decreasing cell surface hydrophobicity. These results position the KRN7000 analogue as a promising candidate for developing glycolipid-based antibiofilm agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Inès M, Dhouha G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr Res. 2015;416:59–69.
Shu Q, Lou H, Wei T, Liu X, Chen Q. Contributions of glycolipid biosurfactants and glycolipid-modified materials to antimicrobial strategy: a review. Pharmaceutics. 2021;13:227.
Firdose A, Maeda T, Sukri MAM, Yasin NHM, Sabturani N, Aqma WS. Antibacterial mechanism of Pseudomonas aeruginosa UKMP14T rhamnolipids against multidrug resistant Acinetobacter baumannii. Microb Pathog. 2024;193:106743.
Ceresa C, Hutton S, Lajarin-Cuesta M, Heaton R, Hargreaves I, Fracchia L, et al. Production of Mannosylerythritol Lipids (MELs) to be Used as Antimicrobial Agents Against S. aureus ATCC 6538. Curr Microbiol. 2020;77:1373–80.
Díaz De Rienzo MA, Banat IM, Dolman B, Winterburn J, Martin PJ. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. N Biotechnol. 2015;32:720–6.
Boxley CJ, Hogan DE, Stolley RM, Maier RM. Synthetic approaches to production of rhamnolipid and related glycolipids. In: Biosurfactants. Elsevier, 2023, pp 251–63.
Mukherji R, Prabhune A. Novel glycolipids synthesized using plant essential oils and their application in quorum sensing inhibition and as antibiofilm agents. Sci World J. 2014;2014:1–7.
Maiti K, Syal K, Chatterji D, Jayaraman N. Synthetic arabinomannan heptasaccharide glycolipids inhibit biofilm growth and augment isoniazid effects in Mycobacterium smegmatis. ChemBioChem. 2017;18:1959–70.
Naresh K, Bharati BK, Avaji PG, Jayaraman N, Chatterji D. Synthetic arabinomannan glycolipids and their effects on growth and motility of the Mycobacterium smegmatis. Org Biomol Chem. 2010;8:592–9.
Perona A, Hoyos P, Ticona LA, García-Oliva C, Merchán A, Hernáiz MJ. Enzymatic synthesis and biological evaluation of glycolipids as potential antibacterial, antibiofilm and antiquorum sensing agents. Catal Today. 2024;433:114623.
Thangarasu AK, Sambyal S, Kumar HMS, Lankalapalli RS. Design, synthesis, and preliminary immunopotentiating activity of new analogues of nojirimycin. Carbohydr Res. 2022;511:108479.
Natori T, Koezuka Y, Higa T. Agelasphins, novel α-galactosylceramides from the marine sponge Agelas mauritianus. Tetrahedron Lett. 1993;34:5591–2.
Banchet-Cadeddu A, Hénon E, Dauchez M, Renault J-H, Monneaux F, Haudrechy A. The stimulating adventure of KRN 7000. Org Biomol Chem. 2011;9:3080.
Vieira ER, da Xisto MIDS, Pele MA, Alviano DS, Alviano CS, Barreto-Bergter E, et al. Monohexosylceramides from rhizopus species isolated from Brazilian caatinga: chemical characterization and evaluation of their antibiofilm and antibacterial activities. Molecules. 2018;23:1331.
Haas R, Gutman J, Wardrip NC, Kawahara K, Uhl W, Herzberg M, et al. Glycosphingolipids enhance bacterial attachment and fouling of nanofiltration membranes. Environ Sci Technol Lett. 2015;2:43–47.
De Gregorio E, Esposito A, Vollaro A, De Fenza M, D’Alonzo D, Migliaccio A, et al. N-Nonyloxypentyl-l-Deoxynojirimycin Inhibits growth, biofilm formation and virulence factors expression of Staphylococcus aureus. Antibiotics. 2020;9:362.
Kozień Ł, Gallienne E, Martin O, Front S, Strus M, Heczko P. PDIA, an iminosugar compound with a wide biofilm inhibitory spectrum covering both gram-positive and gram-negative human bacterial pathogens. Microorganisms. 2022;10:1222.
Cukkemane N, Bikker FJ, Nazmi K, Brand HS, Sotres J, Lindh L, et al. Anti‐adherence and bactericidal activity of sphingolipids against Streptococcus mutans. Eur J Oral Sci. 2015;123:221–7.
Tan LKK, Eccersley LRJ, Sriskandan S. Current views of haemolytic streptococcal pathogenesis. Curr Opin Infect Dis. 2014;27:155–64.
Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A streptococcus. Clin Microbiol Rev. 2014;27:264–301.
Schaffer JN, Pearson MM. Proteus mirabilis and Urinary Tract Infections. Microbiol Spectr 2015; 3. https://doi.org/10.1128/microbiolspec.UTI-0017-2013.
Armbruster CE, Mobley HLT, Pearson MM. Pathogenesis of proteus mirabilis infection. EcoSal Plus 2018; 8. https://doi.org/10.1128/ecosalplus.esp-0009-2017.
Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216–7.
Nucleo E, Fugazza G, Migliavacca R, Spalla M, Comelli M, Pagani L, et al. Differences in biofilm formation and aggregative adherence between β-lactam susceptible and β-lactamases producing P. mirabilis clinical isolates. New Microbiol. 2010;33:37–45.
Wasfi R, Hamed SM, Amer MA, Fahmy LI. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front Cell Infect Microbiol 2020; 10. https://doi.org/10.3389/fcimb.2020.00414.
Pala M, Castelein MG, Dewaele C, Roelants SLKW, Soetaert WK, Stevens CV. Tuning the antimicrobial activity of microbial glycolipid biosurfactants through chemical modification. Front Bioeng Biotechnol 2024; 12. https://doi.org/10.3389/fbioe.2024.1347185.
Jiang Y, Geng M, Bai L. Targeting biofilms therapy: current research strategies and development hurdles. Microorganisms. 2020;8:1222.
Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO. Agents that inhibit bacterial biofilm formation. Future Med Chem. 2015;7:647–71.
Acknowledgements
Financial support from ICMR, India (Grant number: VIR/17/2020/ECD-I) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnakumar, K.A., Remya Babu, R., Sugathan, S. et al. KRN7000 analogues as biofilm disrupting agents against Streptococcus pyogenes and Proteus mirabilis. J Antibiot 78, 246–255 (2025). https://doi.org/10.1038/s41429-025-00810-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-025-00810-1