Abstract
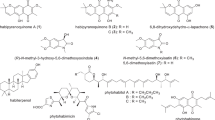
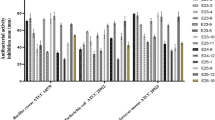
The combined-culture of actinomycetes with mycolic acid-containing bacteria (MACB) Tsukamurella pulmonis TP-B0596 is a promising strategy to produce cryptic metabolites in actinomycetes. In this study, Streptomyces sp. 23-50 was identified as an appropriate strain for co-culturing with T. pulmonis TP-B0596 using on-gel combined-culture screening of 160 strains of actinomycetes. A new pyranonaphthoquinone, actinoquinonal A (1), along with two known congeners, compound 2 and mevashuntin (3), were isolated from the combined-culture of Streptomyces sp. 23-50 with T. pulmonis TP-B0596 based on global natural product social (GNPS) molecular networking. The planar structures of 1–3 were elucidated by analyzing 2D nuclear magnetic resonance (NMR) and LC-MS/MS spectral data, and the absolute configurations of 1 and 3 were unambiguously determined by comparing experimental and calculated ECD spectra. Moreover, the combined-culture characteristic metabolites, including 3, were enhanced when Streptomyces sp. 23-50 was cultured in the presence of pravastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase in the mevalonate pathway, suggesting that T. pulmonis TP-B0596 triggered a shunt in the mevalonate pathway of Streptomyces sp. 23-50. Notably, compounds 1 and 3 exhibited cytotoxicity against human cervical epithelioid carcinoma HeLa S3 (IC50 = 60.5 μM for 1, 0.67 μM for 3) and human colorectal cancer HT29 cells (IC50 = 101.9 μM for 1, 0.45 μM for 3).

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The Supporting Information is available at https://doi.org/10.1038/s41429-025-00821-y. Experimental detail; 1D and 2D NMR spectra, HRMS spectra, and IR spectrum for 1, 1H NMR spectra for 2 and 3, CD spectrum for 3.
References
Kakeya H. Natural products-prompted chemical biology: Phenotypic screening and a new platform for target identification. Nat Prod Rep. 2016;33:648–54.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803.
Lewis K. The Science of antibiotic discovery. Cell. 2020;181:29–45.
Belknap KC, Park CJ, Barth BM, Andam CP. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci Rep. 2020;10:2003.
Navarro-Muñoz JC, Selem-Mojica N, Mullowney MW, Kautsar AA, Tryon JH, Parkinson EI, et al. A computational framework to explore large-scale biosynthetic diversity. Nat Chem Biol. 2020;16:60–68.
van Bergeijk DA, Terlouw BR, Medema MH, van Wezel GP. Ecology and genomics of Actinobacteria: new concepts for natural product discovery. Nat Rev Microbiol. 2020;18:546–58.
Yoshimura A, Saeki R, Nakada R, Tomimoto S, Jomori T, Suganuma K, et al. Membrane-vesicle-mediated interbacterial communication activates silent secondary metabolite production. Angew Chem Int Ed. 2023;62:e202307304.
Kim JH, Lee N, Hwang S, Kim W, Lee Y, Cho S, et al. Discovery of novel secondary metabolites encoded in actinomycete genomes through coculture. J Ind Microbiol Biotechnol. 2021;48:kuaa001.
Onaka H, Mori Y, Igarashi Y, Furumai T. Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl Environ Microbiol. 2011;77:400–6.
Kato M, Asamizu S, Onaka H. Intimate relationships among actinomycetes and mycolic acid-containing bacteria. Sci Rep. 2022;12:7222.
Liu C, Kakeya H. Cryptic chemical communication: Secondary metabolic responses revealed by microbial co-culture. Chem Asian J. 2020;15:327–37.
Sugiyama R, Nishimura S, Ozaki T, Asamizu S, Onaka H, Kakeya H. 5-Alkyl-1,2,3,4-tetrahydroquinolines, new membrane-interacting lipophilic metabolites, produced by combined culture of Streptomyces nigrescens and Tsukamurella pulmonsis. Org Lett. 2015;17:1918–21.
Sugiyama R, Nakatani T, Nishimura S, Takenaka K, Ozaki T, Asamizu S, et al. Chemical interaction of cryptic actinomycete metabolite 5-alkyl-1,2,3,4-tetrahydroquinolines through aggregate formation. Angew Chem Int Ed. 2019;58:13486–91.
Sugiyama R, Nishimura S, Ozaki T, Asamizu S, Onaka H, Kakeya H. Discovery and total synthesis of streptoaminals, antimicrobial [5,5]-spirohemiaminals from the combined-culture of Streptomyces nigrescens and Tsukamurella pulmonis. Angew Chem Int Ed Engl. 2016;55:10278–82.
Jiang YL, Lu S, Hirai G, Kato T, Onaka H, Kakeya H. Enhancement of saccharothriolide production and discovery of a new metabolite, saccharothriolide C2, by combined-culture of Saccharothrix sp. and Tsukamurella pulmonis. Tetrahedron Lett. 2019;60:1072–4.
Jiang YL, Matsumoto T, Kuranaga T, Lu S, Wang WC, Onaka H, et al. Longicatenamides A-D, two diastereomeric pairs of cyclic hexapeptides produced by combined-culture of Streptomyces sp. KUSC_F05 and Tsukamurella pulmonis TP-B0596. J Antibiot. 2021;74:307–16.
Pan C, Ikeda H, Minote M, Tokuda T, Kuranaga T, Taniguchi T, et al. Amoxetamide A, a new anoikis inducer, produced by combined-culture of Amycolatopsis sp. and Tsukamurella pulmonis. J Antibiot. 2024;77:66–70.
Pan CQ, Kuranaga T, Cao X, Suzuki T, Dohmae N, Shinzato N, et al. Amycolapeptins A and B, cyclic nonadepsipeptides produced by combined-culture of Amicolatopsis sp. and Tsukamurella pulmonis. J Org Chem. 2021;86:1843–9.
Wang M, Carver J, Phelan V, Sanchez L, Garg N, Peng Y, et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol. 2016;34:828–37.
Bowden G, Johnson J, Schachtele C. Characterization of Actinomyces with genomic DNA fingerprints and rRNA gene probes. J Dent Res. 1993;72:1171–9.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Kaneko K, Mieda M, Jiang Y, Takahashi N, Kakeya H. Tumescenamide C, a cyclic lipodepsipeptide from Streptomyces sp. KUSC_F05, exerts antimicrobial activity against the scab-forming actinomycete Streptomyces scabiei. J Antibiot. 2024;77:353–64.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, et al. Gaussian 16, Revision C.02. Gaussian, Inc., Wallingford CT, 2019.
Lodewyk MW, Tantillo DJ. Prediction of the structure of nobilisitine A using computed NMR chemical shifts. J Nat Prod. 2011;74:1339–43.
Kulanthaivel P, Perun TJ Jr, Belvo MD, Strobel RL, Paul DC, Williams DC. Novel naphthoquinones from a Streptomyces sp. J Antibiot. 1999;52:256–62.
Shin-ya K, Umeda Y, Chijiwa S, Furihata K, Hayakawa Y, Seto H. Mevashuntin, a novel metabolite produced by inhibition of the mevalonate pathway in Streptomyces prunicolor. Tetrahedron Lett. 2005;46:1273–6.
Acknowledgements
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H06401, 19H02840, 22H04901, 23H04882, and 24H00493; all awarded to H. Kakeya), the Project for Promotion of Cancer Research and Therapeutic Evolution (JP24ama221540 and JP25ama221540 to H. Kakeya), and the Platform Project for Supporting Drug Discovery and Life Science Research (JP24ama121034 and JP25ama121034 to H. Kakeya) from the Japan Agency for Medical Research and Development (AMED), Japan. HOKUSAI (RIKEN) provided the computer resources for the DFT calculations. We acknowledge Ms. Mikiko Ito (RIKEN) for assistance with the computational work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yanagisawa, K., Kaneko, K., Ikeda, H. et al. A new pyranonaphthoquinone, actinoquinonal A, and its congeners from the combined-culture of Streptomyces sp. 23–50 and Tsukamurella pulmonis TP-B0596. J Antibiot 78, 350–358 (2025). https://doi.org/10.1038/s41429-025-00821-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-025-00821-y
This article is cited by
-
Unlocking Streptomyces biosynthetic gene clusters: bioelicitors, co-culture, and beyond
Systems Microbiology and Biomanufacturing (2026)