Abstract
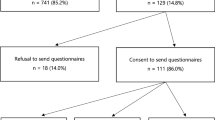
In patients with early breast cancer, personal and tumour characteristics other than family history are increasingly used to prompt genetic testing to guide women’s cancer management (treatment-focused genetic testing, ‘TFGT’). Women without a known strong family history of breast and/or ovarian may be more vulnerable to psychological sequelae arising from TFGT. We compared the impact of TFGT in women with (FH+) and without (FH−) a strong family history on psychological adjustment and surgical decisions. Women aged <50 years with high-risk features were offered TFGT before definitive breast cancer surgery and completed self-report questionnaires at four time points over 12 months. All 128 women opted for TFGT. TFGT identified 18 carriers of a disease-causing variant (50.0% FH+) and 110 non-carriers (59.1% FH+). There were no differences based on family history in bilateral mastectomy (BM) uptake, p = .190, or uptake of risk-reducing bilateral salpingo-oophorectomy (RRBSO), p = .093. FH− women had lower decreases in anxiety a year after diagnosis, p = .011, and regret regarding their decision whether to undergo BM, p = .022, or RRBSO, p = .016 than FH + women. FH− carriers reported significantly higher regret regarding their TFGT choice (p = .024) and test-related distress (p = .012) than FH + carriers, but this regret/distress could not be attributed to a concern regarding a possible worse prognosis. These findings indicate that FH− women may require additional counselling to facilitate informed decisions. Carriers without a family history may require additional follow-up counselling to facilitate psychological adjustment to their positive variant results, extra support in making surgical decisions, and counselling about how best to communicate results to family members.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Trainer A, Lewis C, Tucker K, Meiser B, Friedlander M, Ward R. Treatment-focused genetic assessment in breast cancer - the evolving role of the familial cancer services. Nat Rev Clin Oncol. 2010;7:708–17.
Metcalfe K, Lynch H, Ghadirian P, et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Tr. 2011;127:287–96.
van den Broek A, van’t Veer L, Hooning M, et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J Clin Oncol. 2015;34:409–18.
Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014;348:226.
Evans D, Ingham S, Baildam A, et al. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat. 2013;140:135–42.
Rebbeck T, Kauff N, Domchek S, Meta-analysis of risk reduction estimates associated with risk-reducing salpingooophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87.
Kotsopoulos J, Huzarski T, Gronwald J, et al. Bilateral oophorectomy and breastcancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109:djw177
Telli M, Jensen K, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation–associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33:1895–901.
Moller P, Hagen A, Apold J, et al. Genetic epidemiology of BRCA mutations - family history detects less than 50% of the mutation carriers. Eur J Cancer. 2007;43:1713–7.
Fackenthal J, Olopade O. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7:937–48.
Bradbury A, Patrick-Miller L, Long J, et al. Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genet Med. 2015;17:485–92.
Hilgart J, Coles B, Iredale R. Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database Syst Rev. 2012;2:CD003721.
Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009;28:510–8.
Schlich-Bakker KJ, Warlam-Rodenhuis CC, van Echtelt J, van den Bout J, Ausems MG, ten Kroode HF. Short term psychological distress in patients actively approached for genetic counselling after diagnosis of breast cancer. Eur J Cancer. 2006;42: 2722–8.
Schlich-Bakker KJ, Ausems MG, Schipper M, Ten Kroode HF, Warlam-Rodenhuis CC, BRCA1/2 mutation testing in breast cancer patients: a prospective study of the long-term psychological impact of approach during adjuvant radiotherapy. Breast Cancer Res Treat. 2008;109:507–14.
Shkedi-Rafid S, Gabai-Kapara E, Grinshpun-Cohen J, Levy-Lahad E. BRCA genetic testing of individuals from families with low prevalence of cancer: experiences of carriers and implications for population screening. Genet Med. 2012;14:688–94.
Manchanda R, Loggenberg K, Sanderson S, et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish Community: a randomized controlled trial. J Natl Cancer Inst. 2015;107:379.
Marteau TM, Croyle RT. Psychological responses to genetic testing. Br Med J. 1998;316:693–6.
Watts K, Meiser B, Mitchell G, et al. How should we discuss genetic testing with women newly diagnosed with breast cancer? Design and implementation of a randomized controlled trial of two models of delivering education about treatment-focused genetic testing to younger women newly diagnosed with breast cancer. BMC Cancer. 2012;12:320.
Quinn V, Meiser B, Kirk J, et al. Streamlined education is effective for women newly diagnosed with breast cancer considering genetic testing. Genet Med. 2017;19:448–56.
National Breast Cancer Centre. Advice about familial aspects of breast cancer and epithelial ovarian cancer: A guide for health professionals. Sydney: NHMRC National Breast and Ovarian Cancer Centre; 2010.
Thewes B, Meiser B, Hickie I. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psycho-Oncol. 2001;10:459–68.
Ibbotson T, Maguire P, Selby P, Priestman T, Wallace L. Screening for anxiety and depression in cancer patients: the effects of disease and treatment. Eur J Cancer. 1994;30A:37–40.
Cella D, Chang C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: The multidimensional impact of cancer risk assessment (MICRA) questionnaire. Health Psychol. 2002;21:564–72.
Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Dec Mak. 2003;23:281–92.
Lannin D, Wang S. Are small breast cancers good because they are small or small because they are good? N Engl J Med. 2017;376:2286–91.
Singer J, Willett J. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003.
Cancer Australia. Recommendations for the management of early breast cancer in women with an identified BRCA1 or BRCA2 gene mutation or at high risk of a gene mutation. in: Australia C, editor. A clinical practive guideline. Canberra: Cancer Australia; 2014.
Meiser B, Quinn V, Gleeson M, et al. When knowledge of a heritable gene mutation comes out of the blue: treatment-focused genetic testing in women newly diagnosed with breast cancer. Eur J Hum Genet. 2016;24:1571–1523.
Schwartz M, Lerman C, Brogan B, et al. Impact of BRCA1/2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22:1823–9.
Acknowledgements
We thank the patients who generously participated in this study. We also gratefully acknowledge the assistance of the staff who were involved at each site. Thank you also to Mariana Souza for research assistance. We also acknowledge the support and endorsement of this project by the Psycho-oncology Cooperative Research Group (PoCoG). This trial was funded by a Priority-Driven Collaborative Research Grant, which was jointly supported by Cancer Australia, Cancer Council Australia and the National Breast Cancer Foundation (ID 630405). Bettina Meiser was supported by a Career Development Fellowship Award Level 2 (ID 1003921) from the National Health and Medical Research Council (NHMRC) of Australia and an NHMRC Senior Research Fellowship Level B (ID 1078523). Michelle Peate is currently supported by a National Breast Cancer Foundation Early Career Fellowship (ECF-15-005).
TFGT Collaborative Group
The additional members of the Treatment FocusedGenetic Testing Collaborative Group are: Cabrini Health Melbourne (P. Gregory, L.Lipton, L. McKay, J. Senior); Department of Medical Oncology, Prince of WalesHospital (P. Crowe, A. Matthews, G. Neil, A. Parasyn, D. Thomson); HereditaryCancer Clinic, Prince of Wales Hospital, Sydney (J. Duffy, L. Andrews, J. Gale);Monash Medical Centre, Melbourne (J. Fox, S. Hart, C. Smythe, M. White);Nambour Hospital, Nambour (L. Creighton, J. D’arcy, S. Grieve, E. Secomb); PeterMacCallum Cancer Centre, Melbourne (M. Henderson, J. O’Brien, C. Poliness);Royal Brisbane Hospital (A. Hattam, R. Susman, O. Ung,); Royal North ShoreHospital, Sydney (R. Dickson, K. Moore); St George Hospital, Sydney (P. Bastick, S.Inder, J. Lynch, P. Schwartz, R. Zia); The Poche Centre, Sydney (C. Mak, K. Snook,A. Spillane); University of Melbourne (J. Hopper); Westmead Hospital, Sydney (M.Bowman, D. Cheung, S. Edirimanne, E. Edwards, E. Elder, J. French, D. Moon).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
BM, KT and MG have remunerated consultant roles with the company Astrazeneca with respect to unrelated projects. The remaining authors declare that they have no competing intersts.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The additional members of the Treatment Focused Genetic Testing Collaborative Group are listed at the end of this paper.
Bettina Meiser and Veronica F. Quinn contributed equally to this work.
Rights and permissions
About this article
Cite this article
Meiser, B., Quinn, V.F., Mitchell, G. et al. Psychological outcomes and surgical decisions after genetic testing in women newly diagnosed with breast cancer with and without a family history. Eur J Hum Genet 26, 972–983 (2018). https://doi.org/10.1038/s41431-017-0057-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-017-0057-3
This article is cited by
-
Germline variant spectrum of hereditary cancer susceptibility genes in a Chinese cohort
Human Genomics (2025)
-
The MyCancerGene study: a hybrid type 1 effectiveness-implementation randomized study comparing a patient-centered digital genetic health portal to usual care after receipt of cancer genetic testing
BMC Cancer (2025)
-
Heterogeneity of posttraumatic stress, depression, and fear of cancer recurrence in breast cancer survivors: a latent class analysis
Journal of Cancer Survivorship (2023)
-
Impact of rapid genetic testing for BRCA1 and BRCA2 at time of breast cancer diagnosis on psychosocial functioning
Breast Cancer Research and Treatment (2022)