Abstract
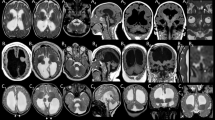
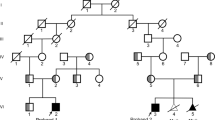
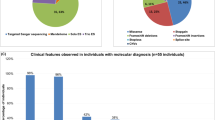
An intact and dynamic microtubule cytoskeleton is crucial for the development, differentiation, and maintenance of the mammalian cortex. Variants in a host of structural microtubulin-associated proteins have been identified to cause a wide spectrum of malformations of cortical development and alterations of microtubule dynamics have been recognized to cause or contribute to progressive neurodegenerative disorders. TBCD is one of the five tubulin-specific chaperones and is required for reversible assembly of the α-/β-tubulin heterodimer. Recently, variants in TBCD, and one other tubulin-specific chaperone, TBCE, have been identified in patients with distinct progressive encephalopathy with a seemingly broad clinical spectrum. Here, we report the clinical, neuroradiological, and neuropathological features in eight patients originating from the Faroe Islands, who presented with an early onset, progressive encephalopathy with features of primary neurodegeneration, and a homogenous clinical course. These patients were homozygous for a TBCD missense variant c.[3099C>G]; p.(Asn1033Lys), which we show has a high carrier frequency in the Faroese population (2.6%). The patients had similar age of onset as the previously reported patients (n = 24), but much shorter survival, which could be caused by either differences in supportive treatment, or alternatively, that shorter survival is intrinsic to the Faroese phenotype. We present a detailed description of the neuropathology and MR imaging characteristics of a subset of these patients, adding insight into the phenotype of TBCD-related encephalopathy. The finding of a Faroese founder variant will allow targeted genetic diagnostics in patients of Faroese descent as well as improved genetic counseling and testing of at-risk couples.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kapitein LC, Hoogenraad CC. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87:492–506.
Francis JW, Goswami D, Novick SJ, et al. Nucleotide binding to ARL2 in the TBCD ∙ ARL2 ∙ β-tubulin complex drives conformational changes in β-tubulin. J Mol Biol. 2017;429:3696–716.
Tischfield MA, Cederquist GY, Gupta ML, Engle EC. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr Opin Genet Dev. 2011;21:286–94.
Breuss MW, Leca I, Gstrein T, Hansen AH, Keays DA. Tubulins and brain development—the origins of functional specification. Mol Cell Neurosci. 2017;84:58–67.
Curiel J, Bey GR, Takanohashi A, et al. TUBB4A mutations result in specific neuronal and oligodendrocytic defects that closely match clinically distinct phenotypes. Hum Mol Genet. 2017;26:4506–18.
Smith BN, Ticozzi N, Fallini C, et al. Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron. 2014;84:324–31.
Sferra A, Baillat G, Rizza T, et al. TBCE mutations cause early-onset progressive encephalopathy with distal spinal muscular atrophy. Am J Hum Genet. 2016;99:974–83.
Dubey J, Ratnakaran N, Koushika SP. Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci. 2015;9. https://doi.org/10.3389/fncel.2015.00343.
Flex E, Niceta M, Cecchetti S, et al. Biallelic mutations in TBCD, encoding the tubulin folding cofactor D, perturb microtubule dynamics and cause early-onset encephalopathy. Am J Hum Genet. 2016;99:962–73.
Miyake N, Fukai R, Ohba C, et al. Biallelic TBCD mutations cause early-onset neurodegenerative encephalopathy. Am J Hum Genet. 2016;99:950–61.
Pode-Shakked B, Barash H, Ziv L, et al. Microcephaly, intractable seizures and developmental delay caused by biallelic variants in TBCD: further delineation of a new chaperone-mediated tubulinopathy. Clin Genet. 2017;91:725–38.
Ikeda T, Nakahara A, Nagano R, et al. TBCD may be a causal gene in progressive neurodegenerative encephalopathy with atypical infantile spinal muscular atrophy. J Hum Genet. 2017;62:473–80.
Edvardson S, Tian G, Cullen H, et al. Infantile neurodegenerative disorder associated with mutations in TBCD, an essential gene in the tubulin heterodimer assembly pathway. Hum Mol Genet. 2016;25:4635–8.
Ostergaard E, Joensen F, Sundberg K, et al. A novel RNASEH2B splice site mutation responsible for Aicardi-Goutieres syndrome in the Faroe Islands. Acta Paediatr Int J Paediatr 2012;101. https://doi.org/10.1111/j.1651-2227.2012.02807.x.
Ostergaard E, Duno M, Batbayli M, Vilhelmsen K, Rosenberg T. A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Mol Vis. 2011;17:1485–92.
Ostergaard E, Hansen FJ, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130:853–61.
Acknowledgements
We thank the families for their participation and Rikke Kammersgaard Nysted and Helle Nørreskov Jensen for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Grønborg, S., Risom, L., Ek, J. et al. A Faroese founder variant in TBCD causes early onset, progressive encephalopathy with a homogenous clinical course. Eur J Hum Genet 26, 1512–1520 (2018). https://doi.org/10.1038/s41431-018-0204-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-018-0204-5
This article is cited by
-
Biallelic pathogenic variants in TBCD-related neurodevelopment disease with mild clinical features
Neurological Sciences (2019)