Abstract
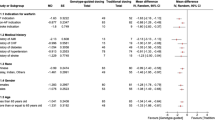
The purpose of this study was to investigate influence of gene polymorphisms of APOB and APOE on risk of bleeding complications at therapeutic INR, during warfarin treatment in Korean patients with mechanical cardiac valves. The study included 142 patients from the EwhA-Severance Treatment Group (EAST) of Warfarin. A total of 12 SNPs was investigated. Five SNPs of APOB (c.13013G>A, c.1853C>T, c.1594C>T, c.293C>T, and c.7545C>T) and five SNPs of APOE (g.4798T>G, g.6406G>A, g.10413T>C, c.388T>C, and c.526C>T) were selected. In addition to selected SNPs, VKORC1 g.6399C>T, and CYP2C9 c.1075A>C, which were known to have significant effects on warfarin stable doses, were also included in the study. Two SNPs of APOB (c.293C>T and c.1853C>T) were associated with bleeding complications. T allele carriers of c.293C>T had 8.6 times (95% CI 2.9–25.5, p < 0.001) increased risk of bleeding, and attributable risk was 88.3%. C allele carriers of c.1853C>T had 6.4 times (95% CI 2.3–17.9, p < 0.001) increased risk of bleeding after adjusting for covariates (attributable risk of 84.3%). AUROC values of models that included c.1853C>T and c.293C>T were 0.771 and 0.802, respectively. Among demographic characteristics, age was the only significant factor. This study revealed that APOB was associated with bleeding complications in patients with warfarin treatment after mechanical cardiac valves.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nishimura R, Otto C, Bonow R, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of Cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:2440–92.
Puri D, Kumar A, Basu R, Chaudhary A, Sarwal V, Sahoo M, et al. Early anticoagulation after mechanical valve implantation, and related complications. J Heart Valve Dis. 2008;17:418–24.
Grzymala-Lubanski B, Svensson P, Renlund H, Jeppsson A, Själander A. Warfarin treatment quality and prognosis in patients with mechanical heart valve prosthesis. Heart. 2017;103:198–203.
Cannegieter S, Rosendaal F, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635.
Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin—fourth edition. Br J Haematol. 2011;154:311–24.
Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017;102:397–404.
Ferder NS, Eby CS, Deych E, Harris JK, Ridker PM, Milligan PE, et al. Ability of VKORC1 and CYP2C9 to predict therapeutic warfarin dose during the initial weeks of therapy. J Thromb Haemost. 2010;8:95–100.
Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D’Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian study on complications of oral anticoagulant therapy. Lancet. 1996;348:423–8.
Abdelhafiz AH, Wheeldon NM. Risk factors for bleeding during anticoagulation of atrial fibrillation in older and younger patients in clinical practice. Am J Geriatr Pharmacother. 2008;6:1–11.
Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis. 2013;35:312–9.
Yang J, Chen Y, Li X, Wei X, Chen X, Zhang L, et al. Influence of CYP2C9 and VKORC1 genotypes on the risk of hemorrhagic complications in warfarin-treated patients: a systematic review and meta-analysis. Int J Cardiol. 2013;168:4234–43.
Korporaal S, Relou I, van Eck M, Strasser V, Bezemer M, Gorter G, et al. Binding of low-density lipoprotein to platelet apolipoprotein E receptor 2 results in phosphorylation of p38MAPK. J Biol Chem. 2004;279:52526–34.
Riddell D, Owen J. Inhibition of ADP-induced platelet aggregation by APOE is not mediated by membrane cholesterol depletion. Thromb Res. 1996;81:597–606.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47.
Barrett J, Fry B, Maller J, Daly M. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
Ward L, Kellis M. HaploRegv4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016;44:D877–81.
Hu Z, Zhang L, Yang Q. Effect of APOB polymorphism on plasma lipid levels and cerebral hemorrhage in Changsha Han Chinese. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:494–9.
Utermann G. Apolipoprotein E polymorphism in health and disease. Am Heart J. 1987;113:433–40.
Lindbohm J, Korja M, Jousilahti P, Salomaa V, Kaprio J. Adverse lipid profile elevates risk for subarachnoid hemorrhage: a prospective population-based cohort study. Atherosclerosis. 2018;274:112–9.
Valappil A, Chaudhary N, Praveenkumar R, Gopalakrishnan B, Girija A. Low cholesterol as a risk factor for primary intracerebral hemorrhage: a case-control study. Ann Indian Acad Neurol. 2012;15:19–22.
Tanaka H, Ueda Y, Hayashi M, Date C, Baba T, Yamashita H, et al. Risk factors for cerebral hemorrhage and cerebral infarction in a Japanese rural community. Stroke. 1982;13:62–73.
Shimamoto T, Komachi Y, Inada H, Doi M, Iso H, Sato S, et al. Trends for coronary heart disease and stroke and their risk factors in Japan. Circulation. 1989;79:503–15.
Korja M, Silventoinen K, Laatikainen T, Jousilahti P, Salomaa V, Hernesniemi J, et al. Risk factors and their combined effects on the incidence rate of subarachnoid hemorrhage—a population-based cohort study. PLoS ONE. 2013;8:e73760.
Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke. 2012;43:1768–74.
Chatterton J, Schlapfer P, Butler E, Gutierrez MM, Puppione DL, Pullinger CR, et al. Identification of apolipoprotein B100 polymorphisms that affect low-density lipoprotein metabolism: description of a new approach involving monoclonal antibodies and dynamic light scattering. Biochemistry. 1995;34:9571–80.
Zhou Y, Mägi R, Milani L, Lauschke V. Global genetic diversity of human apolipoproteins and effects on cardiovascular disease risk. J Lipid Res. 2018;59:1987–2000.
Benn M, Stene M, Nordestgaard B, Jensen GB, Steffensen R, Tybjaerg-Hansen A. Common and rare alleles in apolipoprotein B contribute to plasma levels of low-density lipoprotein cholesterol in the general population. J Clin Endocrinol Metab. 2008;93:1038–45.
Zhang L, Yang QD, Zeng Y. Positive association of apolipoprotein B gene C7673T polymorphism with cerebral hemorrhage with family history. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25:145–9.
Falcone G, Radmanesh F, Brouwers H, Battey TW, Devan WJ, Valant V, et al. APOE ε variants increase risk of warfarin-related intracerebral hemorrhage. Neurology. 2014;83:1139–46.
He S, Zhang H, Cao Y, Nian F, Chen H, Chen W, et al. Association between apolipoprotein E genotype and warfarin response during initial anticoagulation. Biomed Pharmacother. 2018;101:251–6.
Ma Y, Li Z, Chen L, Li X. Blood lipid levels, statin therapy and the risk of intracerebral hemorrhage. Lipids Health Dis. 2016;15:43.
Kim B, Lee S, Ryu W, Kang BS, Kim CK, Yoon BW. Low level of low-density lipoprotein cholesterol increases hemorrhagic transformation in large artery atherothrombosis but not in cardioembolism. Stroke. 2009;40:1627–32.
Lee Y, Eggen J, Soni V, Drozda K, Nutescu EA, Cavallari LH. Warfarin dose requirements in a patient with the CYP2C9*14 allele. Pharmacogenomics. 2014;15:909–14.
Fang M, Go A, Hylek E, Chang Y, Henault LE, Jensvold NG, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54:1231–6.
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (no. 2017R1D1A1B03034033).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yee, J., Kim, W., Chang, B.C. et al. APOB gene polymorphisms may affect the risk of minor or minimal bleeding complications in patients on warfarin maintaining therapeutic INR. Eur J Hum Genet 27, 1542–1549 (2019). https://doi.org/10.1038/s41431-019-0450-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-019-0450-1