Abstract
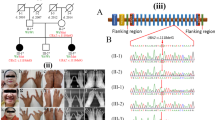
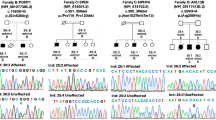
Split-hand/foot malformation (SHFM) is a clinically and genetically heterogeneous condition. We sequentially performed screening of the previously identified Japanese founder 17p13.3 duplication/triplication involving BHLHA9, array comparative genomic hybridization, and whole exome sequencing (WES) in newly recruited 41 Japanese families with non-syndromic and syndromic SHFM. We also carried out WES in seven families with nonsyndromic and syndromic SHFM in which underlying genetic causes including pathogenic copy-number variants (CNVs) remained undetected in our previous studies of 56 families. Consequently, we identified not only known pathogenic CNVs (17p13.3 duplications/triplications [n = 21], 2q31 deletion [n = 1], and 10q24 duplications [n = 3]) and rare variants in known causative genes (TP63 [n = 3], DLX5 [n = 1], IGF2 [n = 1], WNT10B [n = 3], WNT10B/PORCN [n = 1], and PORCN [n = 1]), but also a de novo 19q13.11 deletion disrupting UBA2 (n = 1) and variants that probably affect function in LRP6 (n = 1) and UBA2 (n = 1). Thus, together with our previous data based on testing of 56 families, molecular studies for a total of 97 families with SHFM revealed underlying genetic causes in 75 families, and clinical studies for the 75 families indicated a certain degree of correlation between genetic causes and phenotypes. The results imply that SHFM primarily occurs as a genetic disorder with genotype–phenotype correlations. Furthermore, the results together with previous data such as the development of SHFM in Lrp6 knockout mice, the presence of SHFM in two subjects with 19q13 deletions involving UBA2, and strong mouse Uba2 expression in the developing limb buds, imply that LRP6 and UBA2 represent plausible candidate genes for SHFM.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gurrieri F, Everman DB. Clinical, genetic, and molecular aspects of split-hand/foot malformation: an update. Am J Med Genet A. 2013;161A:2860–72.
Everman DB. Split-hand/foot malformation. In: Stevenson RE, Hall JG, Everman DB, Solomon BD, editors. Human malformations and related anomalies. 3rd ed. Oxford, UK: Oxford University Press; 2016. p. 169–73.
Carter TC, Sicko RJ, Kay DM, Browne ML, Romitti PA, Edmunds ZL, et al. Copy-number variants and candidate gene mutations in isolated split hand/foot malformation. J Hum Genet. 2017;62:877–84.
Nagata E, Kano H, Kato F, Yamaguchi R, Nakashima S, Takayama S, et al. Japanese founder duplications/triplications involving BHLHA9 are associated with split-hand/foot malformation with or without long bone deficiency and Gollop-Wolfgang complex. Orphanet J Rare Dis. 2014;9:125.
Nagata E, Haga N, Fujisawa Y, Fukami M, Nishimura G, Ogata T. Femoral-tibial-digital malformations in a boy with the Japanese founder triplication of BHLHA9. Am J Med Genet A. 2015;167A:3226–8.
Ohtaka K, Fujisawa Y, Takada F, Hasegawa Y, Miyoshi T, Hasegawa T, et al. FGFR1 analyses in four patients with hypogonadotropic hypogonadism with split-hand/foot malformation: implications for the promoter region. Hum Mutat. 2017;38:503–6.
Yamoto K, Saitsu H, Nakagawa N, Nakajima H, Hasegawa T, Fujisawa Y, et al. De novo IGF2 mutation on the paternal allele in a patient with Silver-Russell syndrome and ectrodactyly. Hum Mutat. 2017;38:953–8.
Hiraide T, Nakashima M, Yamoto K, Fukuda T, Kato M, Ikeda H, et al. De novo variants in SETD1B are associated with intellectual disability, epilepsy and autism. Hum Genet. 2018;137:95–104.
Kuzmiak HA, Maquat LE. Applying nonsense-mediated mRNA decay research to the clinic: progress and challenges. Trends Mol Med. 2006;12:306–16.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Nord AS, Lee M, King MC, Walsh T. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genom. 2011;12:184.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8.
Chowdhury S, Bandholz AM, Parkash S, Dyack S, Rideout AL, Leppig KA, et al. Phenotypic and molecular characterization of 19q12q13.1 deletions: a report of five patients. Am J Med Genet A. 2014;164A:62–9.
Abe KT, Rizzo IMPO, Coelho ALV, Sakai N Jr, Carvalho DR, Speck-Martins CE. 19q13.11 microdeletion: clinical features overlapping ectrodactyly ectodermal dysplasia-clefting syndrome phenotype. Clin Case Rep. 2018;6:1300–7.
Costa MW, Lee S, Furtado MB, Xin L, Sparrow DB, Martinez CG, et al. Complex SUMO-1 regulation of cardiac transcription factor Nkx2-5. PLoS One. 2011;6:e24812.
Klopocki E, Lohan S, Doelken SC, Stricker S, Ockeloen CW. Soares et al. Duplications of BHLHA9 are associated with ectrodactyly and tibia hemimelia inherited in non-Mendelian fashion. J Med Genet. 2012;49:119–25.
Zakany J, Duboule D. The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev. 2007;17:359–66.
Dimitrov B, Balikova I, de Ravel T, Van Esch H, De Smedt M, Baten E, et al. 2q31.1 microdeletion syndrome: redefining the associated clinical phenotype. J Med Genet. 2011;48:98–104.
Li CF, Angione K, Milunsky JM. Identification of critical region responsible for split hand/foot malformation type 3 (SHFM3) phenotype through systematic review of literature and mapping of breakpoints using microarray data. Microarrays. 2015;5:E2.
Becic T, Kero D, Vukojevic K, Mardesic S, Saraga-Babic M. Growth factors FGF8 and FGF2 and their receptor FGFR1, transcriptional factors Msx-1 and MSX-2, and apoptotic factors p19 and RIP5 participate in the early human limb development. Acta Histochem. 2018;120:205–14.
Marinić M, Aktas T, Ruf S, Spitz F. An integrated holo-enhancer unit defines tissue and gene specificity of the Fgf8 regulatory landscape. Dev Cell. 2013;24:530–42.
van Bokhoven H, Brunner HG. Splitting p63. Am J Hum Genet. 2002;71:1–13.
Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99:143–53.
Wang X, Xin Q, Li L, Li J, Zhang C, Qiu R, et al. Exome sequencing reveals a heterozygous DLX5 mutation in a Chinese family with autosomal-dominant split-hand/foot malformation. Eur J Hum Genet. 2014;22:1105–10.
Begemann M, Zirn B, Santen G, Wirthgen E, Soellner L, Büttel HM, et al. Paternally inherited IGF2 mutation and growth restriction. N Engl J Med. 2015;373:349–56.
Eggermann T, Begemann M, Binder G, Spengler S. Silver-Russell syndrome: genetic basis and molecular genetic testing. Orphanet J Rare Dis. 2010;5:19.
Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer EM, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–94.
Hardouin SN, Guo R, Romeo PH, Nagy A, Aubin JE. Impaired mesenchymal stem cell differentiation and osteoclastogenesis in mice deficient for Igf2-P2 transcripts. Development. 2011;138:203–13.
Bostwick B, Fang P, Patel A, Sutton VR. Phenotypic and molecular characterization of focal dermal hypoplasia in 18 individuals. Am J Med Genet C Semin Med Genet. 2016;172C:9–20.
Allache R, Lachance S, Guyot MC, De Marco P, Merello E, Justice MJ, et al. Novel mutations in Lrp6 orthologs in mouse and human neural tube defects affect a highly dosage-sensitive Wnt non-canonical planar cell polarity pathway. Hum Mol Genet. 2014;23:1687–99.
Ockeloen CW, Khandelwal KD, Dreesen K, Ludwig KU, Sullivan R. van Rooij IALM, et al. Novel mutations in LRP6 highlight the role of WNT signaling in tooth agenesis. Genet Med. 2016;18:1158–62.
Massink MP, Créton MA, Spanevello F, Fennis WM, Cune MS, Savelberg SM, et al. Loss-of-function mutations in the WNT co-receptor LRP6 cause autosomal-dominant oligodontia. Am J Hum Genet. 2015;97:621–6.
Sukenik Halevy R, Chien HC, Heinz B, Bamshad MJ, Nickerson DA. Mutations in the fourth β-propeller domain of LRP4 are associated with isolated syndactyly with fusion of the third and fourth fingers. Hum Mutat. 2018;39:811–5.
Venegas-Vega C, Nieto-Martínez K, Martínez-Herrera A, Gómez-Laguna L, Berumen J, Cervantes A. et al. 19q13.11 microdeletion concomitant with ins(2;19)(p25.3; q13.1q13.4)dn in a boy: potential role of UBA2 in the associated phenotype. Mol Cytogenet. 2014;7:61.
Melo JB, Estevinho A, Saraiva J, Ramos L, Carreira IM. Cutis Aplasia as a clinical hallmark for the syndrome associated with 19q13.11 deletion: the possible role for UBA2 gene. Mol Cytogenet. 2015;8:21.
Marble M, Guillen Sacoto MJ, Chikarmane R, Gargiulo D, Juusola J. Missense variant in UBA2 associated with aplasia cutis congenita, duane anomaly, hip dysplasia and other anomalies: a possible new disorder involving the SUMOylation pathway. Am J Med Genet A. 2017;173:758–61.
Ghioni P, D’Alessandra Y, Mansueto G, Jaffray E, Hay RT, La Mantia G, et al. The protein stability and transcriptional activity of p63alpha are regulated by SUMO-1 conjugation. Cell Cycle. 2005;4:183–90.
Ranieri M, Vivo M, De Simone M, Guerrini L, Pollice A, La Mantia G, et al. Sumoylation and ubiquitylation crosstalk in the control of ΔNp63α protein stability. Gene. 2018;645:34–40.
Williams TJ, Pepitone ME, Christensen SE, Cooke BM, Huberman AD, Breedlove NJ, et al. Finger-length ratios and sexual orientation. Nature. 2000;404:455–6.
Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–80.
Acknowledgements
We are grateful to all patients and their parents for their cooperation. We thank Ms. Aya Kitamoto and Mr. Naoki Adachi for their technical support. We also thank Drs Toshiro Nagai, Hironao Numabe, Seiji Mizuno, Toshiro Nagai, Keisuke Nagasaki, Hiroaki Yagasaki, You Omura, Noriyuki Namba, Satoshi Okada, Sadao Ito, Hiromi Iwata, Yuri Endo, Kaori Hara, Takahito Wada, and Akira Oishi for providing clinical information and patient samples.
Funding
This work was supported by Grants from the Japan Agency for Medical Research and Development (AMED) (JP17ek0109297 and JP18ek0109301) and by Grant-in-Aid for Scientific Research on Innovative Areas (JP17H06428) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board Committee of Hamamatsu University School of Medicine, and was performed after obtaining written informed consent (IC) from adult subjects (≥20 years old) or parents of nonadult subjects (<20 years old). We also obtained informed assent from children aged ≥6 years.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yamoto, K., Saitsu, H., Nishimura, G. et al. Comprehensive clinical and molecular studies in split-hand/foot malformation: identification of two plausible candidate genes (LRP6 and UBA2). Eur J Hum Genet 27, 1845–1857 (2019). https://doi.org/10.1038/s41431-019-0473-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-019-0473-7
This article is cited by
-
Rare phenotype: Hand preaxial polydactyly associated with LRP6-related tooth agenesis in humans
npj Genomic Medicine (2021)
-
Genome sequencing in families with congenital limb malformations
Human Genetics (2021)