Abstract
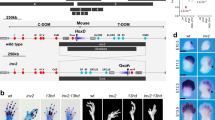
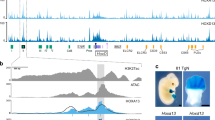
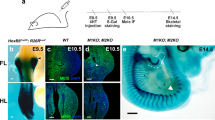
The HoxD cluster is critical for vertebrate limb development. Enhancers located in both the telomeric and centromeric gene deserts flanking the cluster regulate the transcription of HoxD genes. In rare patients, duplications, balanced translocations or inversions misregulating HOXD genes are responsible for mesomelic dysplasia of the upper and lower limbs. By aCGH, whole-genome mate-pair sequencing, long-range PCR and fiber fluorescent in situ hybridization, we studied patients from two families displaying mesomelic dysplasia limited to the upper limbs. We identified microduplications including the HOXD cluster and showed that microduplications were in an inverted orientation and inserted between the HOXD cluster and the telomeric enhancers. Our results highlight the existence of an autosomal dominant condition consisting of isolated ulnar dysplasia caused by microduplications inserted between the HOXD cluster and the telomeric enhancers. The duplications likely disconnect the HOXD9 to HOXD11 genes from their regulatory sequences. This presumptive loss-of-function may have contributed to the phenotype. In both cases, however, these rearrangements brought HOXD13 closer to telomeric enhancers, suggesting that the alterations derive from the dominant-negative effect of this digit-specific protein when ectopically expressed during the early development of forearms, through the disruption of topologically associating domain structure at the HOXD locus.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201.
Dollé P, Izpisúa-Belmonte JC, Falkenstein H, Renucci A, Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989;342:767–72.
Kmita M, Duboule D. Organizing axes in time and space; 25 years of collinear tinkering. Science. 2003;301:331–3.
Montavon T, Soshnikova N, Mascrez B, Joye E, Thevenet L, Splinter E, et al. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147:1132–45.
Tarchini B, Duboule D. Control of Hoxd genes’ collinearity during early limb development. Dev Cell. 2006;10:93–103.
Zakany J, Duboule D. The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev. 2007;17:359–66.
Gonzalez F, Duboule D, Spitz F. Transgenic analysis of Hoxd gene regulation during digit development. Dev Biol. 2007;306:847–59.
Spitz F, Gonzalez F, Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell. 2003;113:405–17.
Andrey G, Montavon T, Mascrez B, Gonzalez F, Noordermeer D, Leleu M, et al. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science. 2013;340:1234167.
Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–80.
Lonfat N, Duboule D. Structure, function and evolution of topologically associating domains (TADs) at HOX loci. FEBS Lett 2015;589:2869–76.
Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, Le Merrer M, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. 2011;155A:943–68.
Reardon W, Hall CM, Slaney S, Huson SM, Connell J, al-Hilaly N, et al. Mesomelic limb shortness: a previously unreported autosomal recessive type. Am J Med Genet. 1993;47:788–92.
Kantaputra PN, Gorlin RJ, Langer LO Jr. Dominant mesomelic dysplasia, ankle, carpal, and tarsal synostosis type: a new autosomal dominant bone disorder. Am J Med Genet. 1992;44:730–7.
Kantaputra PN. Thirteen-year-follow up report on mesomelic dysplasia, Kantaputra type (MDK), and comments on the paper of the second reported family of MDK by Shears et al. Am J Med Genet A. 2004;128A:1–5.
Kwee ML, van de Sluijs JA, van Vugt JM, Wijnaendts LC, Gille JJ. Mesomelic dysplasia, Kantaputra type: clinical report, prenatal diagnosis, no evidence for SHOX deletion/mutation. Am J Med Genet A. 2004;128A:404–9.
Shears DJ, Offiah A, Rutland P, Sirimanna T, Bitner-Glindzicz M, Hall C. Kantaputra mesomelic dysplasia: a second reported family. Am J Med Genet A. 2004;128A:6–11.
Siwicka KA, Kitoh H, Nishiyama M, Ishiguro N. A case of mesomelic dysplasia Kantaputra type–new findings and a new diagnostic approach. J Pediatr Orthop B. 2008;17:271–6.
Kantaputra PN, Klopocki E, Hennig BP, Praphanphoj V, Le Caignec C, Isidor B, et al. Mesomelic dysplasia Kantaputra type is associated with duplications of the HOXD locus on chromosome 2q. Eur J Hum Genet. 2010;18:1310–4.
Cho TJ, Kim OH, Choi IH, Nishimura G, Superti-Furga A, Kim KS, et al. A dominant mesomelic dysplasia associated with a 1.0-Mb microduplication of HOXD gene cluster at 2q31.1. J Med Genet. 2010;47:638–9.
Fryns JP, Hofkens G, Fabry G, van den Berghe H. Isolated mesomelic shortening of the forearm in father and daughter: a new entity in the group of mesomelic dysplasias. Clin Genet. 1988;33:57–9.
Mégarbané A, Ghanem I. Severe autosomal dominant upper-limb mesomelic dysplasia: report of a second family. Clin Genet. 2005;68:567–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods. 2001;25:402–8.
Heiskanen M, Kallioniemi O, Palotie A. Fiber-FISH: experiences and a refined protocol. Genet Anal. 1996;12:179–84.
Park H, Kim JI, Ju YS, Gokcumen O, Mills RE, Kim S, et al. Discovery of common Asian copy number variants using integrated high-resolution array CGH and massively parallel DNA sequencing. Nat Genet. 2010;42:400–5.
Prieur M, Lapierre J, Le Lorch M, Ozilou C, Amiel J, Sanlaville D, et al. HOXD gene cluster haploinsufficiency does not generate gross limb abnormalities. Eur J Hum Genet. 2000;8:74.
Slavotinek A, Schwarz C, Getty JF, Stecko O, Goodman F, Kingston H. Two cases with interstitial deletions of chromosome 2 and sex reversal in one. Am J Med Genet. 1999;86:75–81.
Kmita M, Tarchini B, Zàkàny J, Logan M, Tabin CJ, Duboule D. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435:1113–6.
Dlugaszewska B, Silahtaroglu A, Menzel C, Kübart S, Cohen M, Mundlos S, et al. Breakpoints around the HOXD cluster result in various limb malformations. J Med Genet. 2006;43:111–8.
Ventruto V, Pisciotta R, Renda S, Festa B, Rinaldi MM, Stabile M, et al. Multiple skeletal familial abnormalities associated with balanced reciprocal translocation 2;8(q32; p13). Am J Med Genet. 1983;16:589–94.
Spitz F, Montavon T, Monso-Hinard C, Morris M, Ventruto ML, Antonarakis S, et al. A t(2;8) balanced translocation with breakpoints near the human HOXD complex causes mesomelic dysplasia and vertebral defects. Genomics. 2002;79:493–8.
Ghoumid J, Andrieux J, Sablonnière B, Odent S, Philippe N, Zanlonghi X, et al. Duplication at chromosome 2q31.1-q31.2 in a family presenting syndactyly and nystagmus. Eur J Hum Genet. 2011;19:1198–201.
Lim BC, Min BJ, Park WY, Oh SK, Woo MJ, Choi JS, et al. A unique phenotype of 2q24.3-2q32.1 duplication: early infantile epileptic encephalopathy without mesomelic dysplasia. J Child Neurol. 2014;29:260–4.
Hérault Y, Fraudeau N, Zákány J, Duboule D. Ulnaless (Ul), a regulatory mutation inducing both loss-of-function and gain-of-function of posterior Hoxd genes. Development. 1997;124:3493–500.
Peichel CL, Prabhakaran B, Vogt TF. The mouse Ulnaless mutation deregulates posterior HoxD gene expression and alters appendicular patterning. Development. 1997;124:3481–92.
Duboule D, Morata G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994;10:358–64.
van der Hoeven F, Zákány J, Duboule D. Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell. 1996;85:1025–35.
Peron A, Boito S, Rizzuti T, Borzani I, Baccarin M, Bedeschi MF, et al. Prenatal upper-limb mesomelia and 2q31.1 microdeletions affecting the regulatory genome. Genet Med. 2018;20:1483–4.
Kragesteen BK, Duboule D, Mundlos S, Spielmann M. Response to Peron et al. Genet Med. 2018;20:1481–2.
Acknowledgements
We are grateful to the patients and their families who participated in this study. We are most grateful to the Genomics platform of Nantes (Biogenouest Genomics) core facility for its technical support. DNA panels from the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds) were used in this study.
Author information
Authors and Affiliations
Contributions
Authors’ roles: Study design: CLC, OP, and AT. Study conduct: CLC, OP, and AT. BdC, CB, AM, and AT clinically characterized the patients and collected blood samples. Array CGH analysis and interpretation of the data: CLC, AB, OP, and MSC. Whole-genome sequencing and data interpretation: OP, PL, RR, CSB, and DS. Fiber FISH experiments and data interpretation: OP, MSC. Drafting manuscript: CLC, OP, DD, and AT. Revising manuscript content: CLC, OP, DD, MLV, AM, and AT. Approving final version of manuscript: CLC, OP, AB, BdC, CB, DD, MLV, PL, RR, CSB, MSC, DS, DD, AM, and AT. CLC and AT take responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Le Caignec, C., Pichon, O., Briand, A. et al. Fryns type mesomelic dysplasia of the upper limbs caused by inverted duplications of the HOXD gene cluster. Eur J Hum Genet 28, 324–332 (2020). https://doi.org/10.1038/s41431-019-0522-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-019-0522-2
This article is cited by
-
Structural variants in the 3D genome as drivers of disease
Nature Reviews Genetics (2025)
-
Inhibition of HOXD11 promotes cartilage degradation and induces osteoarthritis development
Journal of Orthopaedic Surgery and Research (2024)
-
Mesomelic dysplasias associated with the HOXD locus are caused by regulatory reallocations
Nature Communications (2021)