Abstract
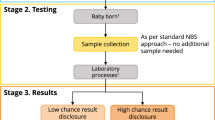
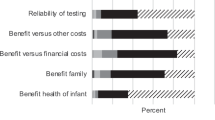
Newborn screening (NBS) is an important part of public healthcare systems in many countries. The provision of information to parents about NBS is now recognised as an integral part of the screening process. Informing parents on all aspects of screening helps to achieve the benefits, promote trust and foster support for NBS. Therefore, policies and guidelines should exist to govern how the information about NBS is provided to parents, taking into account evidence-based best practices. The purpose of our survey was to explore whether any legally binding provisions, guidelines or recommendations existed pertaining to the provision of information about NBS to parents across Europe. Questions were designed to determine the regulatory process of when, by whom and how parents should be informed about screening. Twenty-seven countries participated in the survey. The results indicated that most countries had some sort of legal framework or guidelines for the provision of information to parents. However, only 37% indicated that the provision of information was required prenatally. The majority of countries were verbally informing parents with the aid of written materials postnatally, just prior to sample collection. Information was provided by a neonatologist, midwife or nurse. A website dedicated to NBS was available for 67% of countries and 89% had written materials about NBS for parents. The survey showed that there is a lack of harmonisation among European countries in the provision of information about NBS and emphasised the need for more comprehensive guidelines at the European level.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Martinez-Morillo E, Prieto Garcia B, Alvarez Menendez FV. Challenges for worldwide harmonization of newborn screening programs. Clin Chem. 2016;62:689–98.
Therrell BL Jr., Padilla CD. Barriers to implementing sustainable national newborn screening in developing health systems. Int J Pediatr Adolesc Med. 2014;1:49–60.
Hewlett J, Waisbren SE. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J Inherit Metab Dis. 2006;29:677–82.
Kemper AR, Fant KE, Clark SJ. Informing parents about newborn screening. Public Health Nurs. 2005;22:332–8.
Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–21.
Clayton EW. Talking with parents before newborn screening. J Pediatr. 2005;147:S26–9.
Jansen ME, van den Bosch LJM, Hendriks MJ, Scheffer MMJ, Heijnen ML, Douglas CMW, et al. Parental perspectives on retention and secondary use of neonatal dried bloodspots: a Dutch mixed methods study. BMC Pediatr. 2019;19:230.
Liebl B, Nennstiel-Ratzel U, von Kries R, Fingerhut R, Olgemoller B, Zapf A, et al. Expanded newborn screening in Bavaria: tracking to achieve requested repeat testing. Prev Med. 2002;34:132–7.
Ulph F, Wright S, Dharni N, Payne K, Bennett R, Roberts S, et al. Provision of information about newborn screening antenatally: a sequential exploratory mixed-methods project. Health Technol Assess. 2017;21:1–240.
Cunningham S, O’Doherty KC, Senecal K, Secko D, Avard D. Public concerns regarding the storage and secondary uses of residual newborn bloodspots: an analysis of print media, legal cases, and public engagement activities. J Community Genet. 2015;6:117–28.
Streetly A, Sisodia R, Dick M, Latinovic R, Hounsell K, Dormandy E. Evaluation of newborn sickle cell screening programme in England: 2010-2016. Arch Dis Child. 2018;103:648–53.
Araia MH, Wilson BJ, Chakraborty P, Gall K, Honeywell C, Milburn J, et al. Factors associated with knowledge of and satisfaction with newborn screening education: a survey of mothers. Genet Med. 2012;14:963–70.
Suriadi C, Jovanovska M, Quinlivan JA. Factors affecting mothers’ knowledge of genetic screening. Aust N Z J Obstet Gynaecol. 2004;44:30–4.
Nicholls SG, Southern KW. Parental information use in the context of newborn bloodspot screening. An exploratory mixed methods study. J Community Genet. 2012;3:251–7.
Al-Sulaiman A, Kondkar AA, Saeedi MY, Saadallah A, Al-Odaib A, Abu-Amero KK. Assessment of the knowledge and attitudes of Saudi mothers towards newborn screening. Biomed Res Int. 2015;2015:718674.
Frankova V, Dohnalova A, Peskova K, Hermankova R, O’Driscoll R, Jesina P, et al. Factors influencing parental awareness about newborn screening. Int J Neonat Screen. 2019;5. https://doi.org/10.3390/ijns5030035.
American Academy of Pediatrics. Newborn screening: a blueprint for the future executive summary: newborn screening task force reporg. Pediatrics. 2000;106:386–8.
Loeber JG, Burgard P, Cornel MC, Rigter T, Weinreich SS, Rupp K, et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1. from blood spot to screening result. J Inherit Metab Dis. 2012;35:603–11.
Burgard P, Cornel M, Di Filippo F, Haege G, Hoffmann GF, Lindner M, et al. Report on the practices of newborn screening for rare disorders implemented in member states of the European Union, candidate, potential candidate and EFTA countries. 2012. http://old.iss.it/binary/cnmr/cont/Report_NBS_Current_Practices_20120108_FINAL.pdf. Accessed 17 Sept 2019.
Cornel MC, Rigter T, Weinreich SS, Burgard P, Hoffmann GF, Lindner M, et al. A framework to start the debate on neonatal screening policies in the EU: an expert opinion document. Eur J Hum Genet. 2014;22:12–7.
Fant KE, Clark SJ, Kemper AR. Completeness and complexity of information available to parents from newborn-screening programs. Pediatrics 2005;115:1268–72.
NHS public health functions agreement 2018-19, service specification no.19, NHS newborn blood spot screening programme. https://www.england.nhs.uk/wp-content/uploads/2017/04/Gateway-ref-07840-180913-Service-specification-No.-19-NHS-Newborn-Blood-Spot-Screening.pdf. Accessed 15 Oct 2019.
Davis TC, Humiston SG, Arnold CL, Bocchini JA Jr., Bass PF 3rd, Kennen EM, et al. Recommendations for effective newborn screening communication: results of focus groups with parents, providers, and experts. Pediatrics. 2006;117:S326–40.
Botkin JR, Rothwell E, Anderson RA, Rose NC, Dolan SM, Kuppermann M, et al. Prenatal education of parents about newborn screening and residual dried blood spots: a randomized clinical trial. JAMA Pediatr. 2016;170:543–9.
Serving the family from birth to the medical home. A report from the newborn screening task force convened in Washington DC, May 10-11, 1999. Pediatrics. 2000;106:383–427.
Botkin JR, Rothwell E, Anderson R, Stark L, Goldenberg A, Lewis M, et al. Public attitudes regarding the use of residual newborn screening specimens for research. Pediatrics. 2012;129:231–8.
Charles T, Pitt J, Halliday J, Amor DJ. Implementation of written consent for newborn screening in Victoria, Australia. J Paediatr Child Health. 2014;50:399–404.
Caggana M, Jones EA, Shahied SI, Tanksley S, Hermerath CA, Lubin IM. Newborn screening: from Guthrie to whole genome sequencing. Public Health Rep. 2013;128:14–9.
Hanafin S. Report on the newborn screening card archive forum. Ireland: Department of Health; 2017. p. 1–35. https://assets.gov.ie/41907/635daa08fe744854b1feb6f85d44f0c6.pdf. Accessed 09 Dec 2019.
Australian Health Minsters’ Advisory Council. Newborn Bloodspot Screening, National Policy Framework. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/newborn-bloodspot-screening. Accessed 6 Oct 2019.
Wilson K, Kennedy SJ, Potter BK. Developing a national newborn screening strategy for Canada. Health Law Rev. 2010;18:31–9.
Etchegary H, Nicholls SG, Tessier L, Simmonds C, Potter BK, Brehaut JC, et al. Consent for newborn screening: parents’ and health-care professionals’ experiences of consent in practice. Eur J Hum Genet. 2016;24:1530–4.
Detmar S, Hosli E, Dijkstra N, Nijsingh N, Rijnders M, Verweij M. Information and informed consent for neonatal screening: opinions and preferences of parents. Birth. 2007;34:238–44.
Howard HC, Knoppers BM, Cornel MC, Wright Clayton E, Senecal K, Borry P, et al. Whole-genome sequencing in newborn screening? A statement on the continued importance of targeted approaches in newborn screening programmes. Eur J Hum Genet. 2015;23:1593–600.
Funding
Institutional support was provided by projects LM2018132 from the Large Infrastructure Projects of the Czech Ministry of Education, PROGRES Q26 from Charles University and RVO VFN 64165 from the Ministry of Health, Czech Republic. Several authors of this publication are members of the European Reference Network for Rare Hereditary Metabolic Disorders (MetabERN)—Project ID No. 739543.
Members of the European Society of Human Genetics (ESHG)-EuroGentest Quality Sub-CommitteeZandra C. Deans31, Christi J. van Asperen32 Mick J. Henderson33, David Barton34, Elisabeth M. C. Dequeker35,36, Isabel Marques Carreira37, Thomy de Ravel38, Katrina Rack39, Katrin Õunap40
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Franková, V., Driscoll, R.O., Jansen, M.E. et al. Regulatory landscape of providing information on newborn screening to parents across Europe. Eur J Hum Genet 29, 67–78 (2021). https://doi.org/10.1038/s41431-020-00716-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-00716-6
This article is cited by
-
Genomic sequencing in newborn screening: balancing consent with the right of the asymptomatic at-risk child to be found
European Journal of Human Genetics (2025)
-
A newborn Screening Programme for Inborn errors of metabolism in Galicia: 22 years of evaluation and follow-up
Orphanet Journal of Rare Diseases (2024)
-
Identification of maternal attitudes and knowledge about newborn screenings: a Turkey sample
Journal of Community Genetics (2023)