Abstract
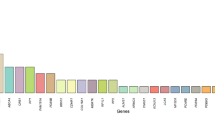
Since a substantial difference in the prevalence of genetic causes of rod-cone dystrophy (RCD) was found among different populations, we conducted a systematic review of the genetic findings associated with RCD in Arab countries. Of the 816 articles retrieved from PubMed, 31 studies conducted on 407 participants from 11 countries were reviewed. Next-generation sequencing (NGS) was the most commonly used technique (68%). Autosomal recessive pattern was the most common pattern of inheritance (97%) and half of the known genes associated with RCD (32/63) were identified. In the Kingdom of Saudi Arabia, in addition to RP1 (20%) and TULP1 (20%), gene defects in EYS (8%) and CRB1 (7%) were also prevalently mutated. In North Africa, the main gene defects were in MERTK (18%) and RLBP1 (18%). Considering all countries, RP1 and TULP1 remained the most prevalently mutated. Variants in TULP1, RP1, EYS, MERTK, and RLBP1 were the most prevalent, possibly because of founder effects. On the other hand, only ten Individuals were found to have dominant or X-linked RCD. This is the first time a catalog of RCD genetic variations has been established in subjects from the Arabi countries. Although the last decade has seen significant interest, expertise, and an increase in RCD scientific publication, much work needs to be conducted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in PubMed repository at https://www.ncbi.nlm.nih.gov/clinvar/submitters/507726.
References
Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40.
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809.
Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genom. 2011;12:238–49.
Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet. 2013;84:132–41.
Dryja TP, Hahn LB, Cowley GS, McGee TL, Berson EL. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:9370–4.
Berson EL, Grimsby JL, Adams SM, McGee TL, Sweklo E, Pierce EA, et al. Clinical features and mutations in patients with dominant retinitis pigmentosa-1 (RP1). Investig Ophthalmol Vis Sci. 2001;42:2217–24.
Payne A, Vithana E, Khaliq S, Hameed A, Deller J, Abu-Safieh L, et al. RP1 protein truncating mutations predominate at the RP1 adRP locus. Investig Ophthalmol Vis Sci. 2000;41:4069–73.
Avila-Fernandez A, Cantalapiedra D, Aller E, Vallespin E, Aguirre-Lamban J, Blanco-Kelly F, et al. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis. 2010;16:2550–8.
Lenassi E, Vincent A, Li Z, Saihan Z, Coffey AJ, Steele-Stallard HB, et al. A detailed clinical and molecular survey of subjects with nonsyndromic USH2A retinopathy reveals an allelic hierarchy of disease-causing variants. Eur J Hum Genet. 2015;23:1318–27.
McGee TL, Seyedahmadi BJ, Sweeney MO, Dryja TP, Berson EL. Novel mutations in the long isoform of the USH2A gene in patients with Usher syndrome type II or non-syndromic retinitis pigmentosa. J Med Genet. 2010;47:499–506.
Audo I, Sahel JA, Mohand-Said S, Lancelot ME, Antonio A, Moskova-Doumanova V, et al. EYS is a major gene for rod-cone dystrophies in France. Hum Mutat. 2010;31:E1406–35.
Barragan I, Abd El-Aziz MM, Borrego S, El-Ashry MF, O’Driscoll C, Bhattacharya SS, et al. Linkage validation of RP25 Using the 10 K genechip array and further refinement of the locus by new linked families. Ann Hum Genet. 2008;72:454–62.
Arai Y, Maeda A, Hirami Y, Ishigami C, Kosugi S, Mandai M, et al. Retinitis pigmentosa with EYS mutations is the most prevalent inherited retinal dystrophy in Japanese populations. J Ophthalmol. 2015;2015:819760.
Oishi M, Oishi A, Gotoh N, Ogino K, Higasa K, Iida K, et al. Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation sequencing. Investig Ophthalmol Vis Sci. 2014;55:7369–75.
Vervoort R, Lennon A, Bird AC, Tulloch B, Axton R, Miano MG, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000;25:462–6.
Fortina P, Al Khaja N, Al Ali MT, Hamzeh AR, Nair P, Innocenti F, et al. Genomics into Healthcare: the 5th Pan Arab Human Genetics Conference and 2013 Golden Helix Symposium. Hum Mutat. 2014;35:637–40.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Metzker ML. Sequencing technologies—the next generation. Nat Rev Genet. 2010;11:31–46.
Audo I, El Shamieh S, Mejecase C, Michiels C, Demontant V, Antonio A, et al. ARL2BP mutations account for 0.1% of autosomal recessive rod-cone dystrophies with the report of a novel splice variant. Clin Genet. 2017;92:109–11.
Audo I, Mohand-Said S, Boulanger-Scemama E, Zanlonghi X, Condroyer C, Demontant V, et al. MERTK mutation update in inherited retinal diseases. Hum Mutat. 2018;39:887–913.
Gerth-Kahlert C, Tiwari A, Hanson JVM, Batmanabane V, Traboulsi E, Pennesi ME, et al. C2orf71 mutations as a frequent cause of autosomal-recessive retinitis pigmentosa: clinical analysis and presentation of 8 novel mutations. Investig Ophthalmol Vis Sci. 2017;58:3840–50.
Patel N, Aldahmesh MA, Alkuraya H, Anazi S, Alsharif H, Khan AO, et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med. 2016;18:554–62.
Patel N, Alkuraya H, Alzahrani SS, Nowailaty SR, Seidahmed MZ, Alhemidan A, et al. Mutations in known disease genes account for the majority of autosomal recessive retinal dystrophies. Clin Genet. 2018;94:554–63.
Habibi I, Chebil A, Falfoul Y, Allaman-Pillet N, Kort F, Schorderet DF, et al. Identifying mutations in Tunisian families with retinal dystrophy. Sci Rep. 2016;6:37455.
Habibi I, Chebil A, Kort F, Schorderet DF, El Matri L. Exome sequencing confirms ZNF408 mutations as a cause of familial retinitis pigmentosa. Ophthalmic Genet. 2017;38:494–7.
Guillonneau X, Piriev NI, Danciger M, Kozak CA, Cideciyan AV, Jacobson SG, et al. A nonsense mutation in a novel gene is associated with retinitis pigmentosa in a family linked to the RP1 locus. Hum Mol Genet. 1999;8:1541–6.
Pierce EA, Quinn T, Meehan T, McGee TL, Berson EL, Dryja TP. Mutations in a gene encoding a new oxygen-regulated photoreceptor protein cause dominant retinitis pigmentosa. Nat Genet. 1999;22:248–54.
Sullivan LS, Heckenlively JR, Bowne SJ, Zuo J, Hide WA, Gal A, et al. Mutations in a novel retina-specific gene cause autosomal dominant retinitis pigmentosa. Nat Genet. 1999;22:255–9.
Audo I, Mohand-Said S, Dhaenens CM, Germain A, Orhan E, Antonio A, et al. RP1 and autosomal dominant rod-cone dystrophy: novel mutations, a review of published variants, and genotype-phenotype correlation. Hum Mutat. 2012;33:73–80.
El Shamieh S, Boulanger-Scemama E, Lancelot ME, Antonio A, Demontant V, Condroyer C, et al. Targeted next generation sequencing identifies novel mutations in RP1 as a relatively common cause of autosomal recessive rod-cone dystrophy. BioMed Res Int. 2015;2015:485624.
Avila-Fernandez A, Corton M, Nishiguchi KM, Munoz-Sanz N, Benavides-Mori B, Blanco-Kelly F, et al. Identification of an RP1 prevalent founder mutation and related phenotype in Spanish patients with early-onset autosomal recessive retinitis. Ophthalmology. 2012;119:2616–21.
Ullah I, Kabir F, Iqbal M, Gottsch CB, Naeem MA, Assir MZ, et al. Pathogenic mutations in TULP1 responsible for retinitis pigmentosa identified in consanguineous familial cases. Mol Vis. 2016;22:797–815.
Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23:236–47.
Khan AO, Bergmann C, Eisenberger T, Bolz HJ. A TULP1 founder mutation, p.Gln301*, underlies a recognisable congenital rod-cone dystrophy phenotype on the Arabian Peninsula. Br J Ophthalmol. 2015;99:488–92.
Strick DJ, Vollrath D. Focus on molecules: MERTK. Exp Eye Res. 2010;91:786–7.
Ge Z, Bowles K, Goetz K, Scholl HP, Wang F, Wang X, et al. NGS-based Molecular diagnosis of 105 eyeGENE((R)) probands with Retinitis Pigmentosa. Sci Rep. 2015;5:18287.
Ostergaard E, Duno M, Batbayli M, Vilhelmsen K, Rosenberg T. A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Mol Vis. 2011;17:1485–92.
Currie-Alder B, Arvanitis R, Hanafi S. Research in Arabic-speaking countries: funding competitions, international collaboration, and career incentives. Sci Publ Policy. 2017;45:74–82.
Wildeman M, van Ophuizen E, den Dunnen JT, Taschner PE. Improving sequence variant descriptions in mutation databases and literature using the Mutalyzer sequence variation nomenclature checker. Hum Mutat. 2008;29:6–13.
Abu-Ameerh M, Mohammad H, Dardas Z, Barham R, Ali D, Bijawi M, et al. Extending the spectrum of CLRN1- and ABCA4-associated inherited retinal dystrophies caused by novel and recurrent variants using exome sequencing. Mol Genet Genom Med. 2020;8:e1123.
Rivera A, White K, Stohr H, Steiner K, Hemmrich N, Grimm T, et al. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67:800–13.
Khan AO. Phenotype-guided genetic testing of pediatric inherited retinal disease in the United Arab Emirates. Retina. 2019;40:1829–37.
Davidson AE, Schwarz N, Zelinger L, Stern-Schneider G, Shoemark A, Spitzbarth B, et al. Mutations in ARL2BP, encoding ADP-ribosylation-factor-like 2 binding protein, cause autosomal-recessive retinitis pigmentosa. Am J Hum Genet. 2013;93:321–9.
Aldahmesh MA, Safieh LA, Alkuraya H, Al-Rajhi A, Shamseldin H, Hashem M, et al. Molecular characterization of retinitis pigmentosa in Saudi Arabia. Mol Vis. 2009;15:2464–9.
Khan AO, Abu-Safieh L. Rod-cone dystrophy with initially preserved visual acuity despite early macular involvement suggests recessive CERKL mutations. Ophthalmic Genet. 2015;36:369–72.
Azab B, Barham R, Ali D, Dardas Z, Rashdan L, Bijawi M, et al. Novel CERKL variant in consanguineous Jordanian pedigrees with inherited retinal dystrophies. Can J Ophthalmol. 2019;54:51–9.
Jalkh N, Guissart C, Chouery E, Yammine T, El Ali N, Farah HA, et al. Report of a novel mutation in CRB1 in a Lebanese family presenting retinal dystrophy. Ophthalmic Genet. 2014;35:57–62.
Hashmi JA, Albarry MA, Almatrafi AM, Albalawi AM, Mahmood A, Basit S. Whole exome sequencing identified a novel single base pair insertion mutation in the EYS gene in a six generation family with retinitis pigmentosa. Congenit Anom. 2018;58:10–5.
Zobor D, Balousha G, Baumann B, Wissinger B. Homozygosity mapping reveals new nonsense mutation in the FAM161A gene causing autosomal recessive retinitis pigmentosa in a Palestinian family. Mol Vis. 2014;20:178–82.
Khan AO, Al Teneiji AM. Homozygous and heterozygous retinal phenotypes in families harbouring IMPG2 mutations. Ophthalmic Genet. 2019;40:247–51.
Mackay DS, Henderson RH, Sergouniotis PI, Li Z, Moradi P, Holder GE, et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol Vis. 2010;16:369–77.
Ksantini M, Lafont E, Bocquet B, Meunier I, Hamel CP. Homozygous mutation in MERTK causes severe autosomal recessive retinitis pigmentosa. Eur J Ophthalmol. 2012;22:647–53.
Nair P, Hamzeh AR, Malik EM, Oberoi D, Al-Ali MT, Bastaki F. Novel PDE6A mutation in an Emirati patient with retinitis pigmentosa. Oman J Ophthalmol. 2017;10:228–31.
Hmani-Aifa M, Benzina Z, Zulfiqar F, Dhouib H, Shahzadi A, Ghorbel A, et al. Identification of two new mutations in the GPR98 and the PDE6B genes segregating in a Tunisian family. Eur J Hum Genet. 2009;17:474–82.
Coppieters F, Van Schil K, Bauwens M, Verdin H, De Jaegher A, Syx D, et al. Identity-by-descent-guided mutation analysis and exome sequencing in consanguineous families reveals unusual clinical and molecular findings in retinal dystrophy. Genet Med. 2014;16:671–80.
Mejecase C, Mohand-Said S, El Shamieh S, Antonio A, Condroyer C, Blanchard S, et al. A novel nonsense variant in REEP6 is involved in a sporadic rod-cone dystrophy case. Clin Genet. 2018;93:707–11.
Katsanis N, Shroyer NF, Lewis RA, Cavender JC, Al-Rajhi AA, Jabak M, et al. Fundus albipunctatus and retinitis punctata albescens in a pedigree with an R150Q mutation in RLBP1. Clin Genet. 2001;59:424–9.
Dessalces E, Bocquet B, Bourien J, Zanlonghi X, Verdet R, Meunier I, et al. Early-onset foveal involvement in retinitis punctata albescens with mutations in RLBP1. JAMA Ophthalmol. 2013;131:1314–23.
Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22:188–91.
Humbert G, Delettre C, Senechal A, Bazalgette C, Barakat A, Arnaud B, et al. Homozygous deletion related to Alu repeats in RLBP1 causes retinitis punctata albescens. Investig Ophthalmol Vis Sci. 2006;47:4719–24.
Al-Rashed M, Abu Safieh L, Alkuraya H, Aldahmesh MA, Alzahrani J, Diya M, et al. RP1 and retinitis pigmentosa: report of novel mutations and insight into mutational mechanism. Br J Ophthalmol. 2012;96:1018–22.
Albarry MA, Hashmi JA, Alreheli AQ, Albalawi AM, Khan B, Ramzan K, et al. Novel homozygous loss-of-function mutations in RP1 and RP1L1 genes in retinitis pigmentosa patients. Ophthalmic Genet. 2019;40:507–13.
Sohocki MM, Sullivan LS, Mintz-Hittner HA, Birch D, Heckenlively JR, Freund CL, et al. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am J Hum Genet. 1998;63:1307–15.
Haddad MF, Khabour OF, Abuzaideh KA, Shihadeh W. Screening for mutations in RPGR and RP2 genes in Jordanian families with X-linked retinitis pigmentosa. Genet Mol Res. 2016;15:gmr7842.
Eisenberger T, Neuhaus C, Khan AO, Decker C, Preising MN, Friedburg C, et al. Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies. PloS One. 2013;8:e78496.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jaffal, L., Joumaa, H., Mrad, Z. et al. The genetics of rod-cone dystrophy in Arab countries: a systematic review. Eur J Hum Genet 29, 897–910 (2021). https://doi.org/10.1038/s41431-020-00754-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-020-00754-0
This article is cited by
-
A novel HPS3 pathogenic nonsense variant associated with Hermansky-Pudlak syndrome type 3 and a platelet dysfunction
Molecular Biology Reports (2026)
-
Neonatal-Onset Developmental Epileptic Encephalopathy due to 2q24.3 Microduplication Associated with Incidental Finding of RLBP1-Related Retinopathy
Indian Journal of Pediatrics (2025)
-
Genotype-phenotype associations in CRB1 bi-allelic patients: a novel mutation, a systematic review and meta-analysis
BMC Ophthalmology (2024)
-
The genetic landscape of inherited retinal dystrophies in Arabs
BMC Medical Genomics (2023)
-
Clinical and genetic spectrums of 413 North African families with inherited retinal dystrophies and optic neuropathies
Orphanet Journal of Rare Diseases (2022)