Abstract
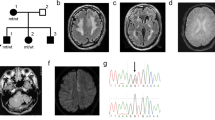
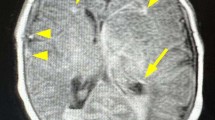
Genetic alterations in COL4A2 are less common than those of COL4A1 and their fetal phenotype has not been described to date. We describe a three-generation family with an intragenic deletion in COL4A2 associated with a prenatal diagnosis of recurrent fetal intracerebral hemorrhage (ICH), and a myriad of cerebrovascular manifestations. Exome sequencing, co-segregation analysis, and imaging studies were conducted on eight family members including two fetuses with antenatal ICH. Histopathological evaluation was performed on the terminated fetuses. An intragenic heterozygous pathogenic in-frame deletion; COL4A2, c.4151_4168del, (p.Thr1384_Gly1389del) was identified in both fetuses, their father with hemiplegic cerebral palsy (CP), as well as other family members. Postmortem histopathological examination identified microscopic foci of heterotopias and polymicrogyria. The variant segregated in affected individuals demonstrating varying degrees of penetrance and a wide phenotypic spectrum including periventricular venous hemorrhagic infarction causing hemiplegic CP, polymicrogyria, leukoencephalopathy, and lacunar stroke. We present radiographic, pathological, and genetic evidence of prenatal ICH and show, for what we believe to be the first time, a human pathological proof of polymicrogyria and heterotopias in association with a COL4A2 disease-causing variant, while illustrating the variable phenotype and partial penetrance of this disease. We highlight the importance of genetic analysis in fetal ICH and hemiplegic CP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Govaert P. Prenatal stroke. Semin Fetal Neonatal Med. 2009;14:250–66.
Bruno CJ, Beslow LA, Witmer CM, Vossough A, Jordan LC, Zelonis S, et al. Haemorrhagic stroke in term and late preterm neonates. Arc Dis Child Fetal Neonatal Ed. 2014;99:48–53.
Jhawar BS, Ranger A, Steven DA, Del Maestro RF. A follow-up study of infants with intracranial hemorrhage at full-term. Can J Neurol Sci. 2005;32:332–9.
Elchalal U, Yagel S, Gomori JM, Porat S, Beni-Adani L, Yanai N, et al. Fetal intracranial hemorrhage (fetal stroke): does grade matter? Ultrasound Obstet Gynecol. 2005;26:233–43.
de Vries LS, Roelants-van Rijn AM, Rademaker KJ, Van Haastert IC, Beek FJ, Groenendaal F. Unilateral parenchymal haemorrhagic infarction in the preterm infant. Eur J Paediatr Neurol. 2001;5:139–49.
Kocaman C, Yilmaz Y. Etiological analysis of presumed perinatal stroke. Brain Dev. 2012;34:133–9.
Takanashi J, Barkovich AJ, Ferriero DM, Suzuki H, Kohno Y. Widening spectrum of congenital hemiplegia: periventricular venous infarction in term neonates. Neurology. 2003;61:531–3.
Haddad J, Messer J, Aranda J. Periventricular haemorrhagic infarction associated with subependymal germinal matrix haemorrhage in the premature newborn. Report of two cases. Eur J Pediatr. 1992;151:63–5.
Kirton A, DeVebere G, Pontigon AM, Macgregor D, Shroff M. Presumed perinatal ischemic stroke: Vascular classification predicts outcomes. Ann Neuro. 2008;63:436–43.
Vermeulen RJ, Peeters-Scholte C, Van Vugt JJ, Barkhof F, Rizzu P, van der Schoor SR, et al. Fetal origin of brain damage in 2 infants with a COL4A1 mutation: fetal and neonatal MRI. Neuropediatrics. 2011;42:1–3.
de Vries LS, Koopman C, Groenendaal F, Van Schoonevel M, Verheijen FW, Verbeek E. et al. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann Neurol. 2009;65:12–8.
Lichtenbelt KD, Pistorius LR, De Tollenaer SM, Mancini GM, De Vries LS. Prenatal genetic confirmation of a COL4A1 mutation presenting with sonographic fetal intracranial hemorrhage. Ultrasound Obstet Gynecol. 2012;39:726–7.
Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, et al. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–5.
van der Knaap MS, Smit LM, Barkhof F, Pijnenburg YA, Zweegman S, Niessen HW, et al. Neonatal porencephaly and adult stroke related to mutations in collagen IV A1. Ann Neurol. 2006;59:504–11.
Dale ST, Coleman LT. Neonatal alloimmune thrombocytopenia: antenatal and postnatal imaging findings in the pediatric brain. AJNR. 2002;23:1457–65.
Sherer DM, Anyaegbunam A, Onyeije C. Antepartum fetal intracranial hemorrhage, predisposing factors and prenatal sonography: a review. Am J Perinatol. 1998;15:431–41.
Hayes B, Ryan S, Stephenson JB, King MD. Cerebral palsy after maternal trauma in pregnancy. Dev Med Child Neurol. 2007;49:700–6.
Yoneda Y, Haginoya K, Kato M, Osaka H, Yokochi K, Arai H, et al. Phenotypic spectrum of COL4A1 mutations: porencephaly to schizencephaly. Ann Neurol. 2013;73:48–57.
Meuwissen ME, de Vries LS, Verbeek HA, Lequin MH, Govaert PP, Schot R, et al. Sporadic COL4A1 mutations with extensive prenatal porencephaly resembling hydranencephaly. Neurology. 2011;76:844–6.
Meuwissen ME, Halley DJ, Smit LS, Lequin MH, Cobben JM, de Coo R, et al. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet Med. 2015;17:843–53.
Vahedi K, Alamowitch S. Clinical spectrum of type IV collagen (COL4A1) mutations: a novel genetic multisystem disease. Curr Opin Neurol. 2011;24:63e8.
Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer JM, et al. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem Cell Biol. 1995;104:267–75.
Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–95.
Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:97–110.
Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–96.
Shah S, Ellard S, Kneen R, Lim M, Osborne N, Rankin J, et al. Childhood presentation of COL4A1 mutations. Developmental Med Child Neurol. 2012;54:569–57.
Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, et al. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–4.
Gould DB, Campbell FP, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in Cola1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71.
Tonk M, Haan J. A review of genetic causes of ischemic and hemorrhagic stroke. J Neurol Sci. 2007;257:273–9.
Jeanne M, Gould DB. Genotype–phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol. 2017;57-58:29–44.
Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhäuser-Klaus A, Pretsch W, et al. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175:725–36.
Yoneda Y, Haginoya K, Arai H, Yamaoka S, Tsurusaki Y, Doi H, et al. De novo and inherited mutations in COL4A2, encoding the type IV collagen a2 chain cause porencephaly. Am J Hum Genet. 2012;90:86–90.
Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, et al. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90:91–101.
Verbeek E, Meuwissen ME, Verheijen FW, Govaert PP, Licht DJ, Kuo DS, et al. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur J Hum Genet. 2012;20:844–51.
Weisz-Hubshman M, Meirson H, Michaelson-Cohen R, Beeri R, Tzur S, Bormans C, et al. Novel WWOX deleterious variants cause early infantile epileptic encephalopathy, severe developmental delay and dysmorphism among Yemenite Jews. Eur J Paediatr Neurol. 2019;23:418–26.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med: Off J Am Coll Med Genet. 2015;17:405–24.
Bove KE. Practice guidelines for autopsy pathology: the perinatal and pediatric autopsy. Autopsy Committee of the College of American Pathologists. Arch Pathol Lab Med. 1997;121:368–76.
Curtis C, Mineyko A, Massicotte P, Leaker M, Jiang XY, Floer A, et al. Thrombophilia risk is not increased in children after perinatal stroke. Blood. 2017;129:2793–800.
Volonghi I, Pezzini A, Del Zotto E, Giossi A, Costa P, Ferrari D, et al. Role of COL4A1 in basement-membrane integrity and cerebral small-vessel disease. The COL4A1 stroke syndrome. Curr Med Chem. 2010;17:1317–24.
Yakovlev PI, Wadsworth RC. Schizencephalies; a study of the congenital clefts in the cerebral mantle; clefts with hydrocephalus and lips separated. J Neuropathol Exp Neurol. 1946;5:169–206.
Labelle-Dumais C, Dilworth DJ, Harrington EP, de Leau M, Lyons D, Kabaeva Z, et al. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 2011;7:e1002062.
Cavallin M, Mine M, Philbert M, Boddaert N, Lepage JM, Coste T, et al. Further refinement of COL4A1 and COL4A2 related cortical malformations. Eur J Med Genet. 2018;61:765–72.
Vitale G, Pichiecchio A, Ormitti F, Tonduti D, Asaro A, Farina L. Cortical malformations and COL4A1 mutation: three new cases. Eur J Paediatr Neurol. 2019;23:410–7.
Raets M, Dudink J, Raybaud C, Ramenghi L, Lequin M, Govaert P. Brain vein disorders in newborn infants. Dev Med Child Neurol. 2015;57:229–40.
Govaert P, Lequin M, Korsten A, Swarte R, Kroon A, Barkovich AJ. Postnatal onset cortical dysplasia associated with infarction of white matter. Brain Res. 2006;1121:250–5.
Acknowledgements
We are grateful to the patients for consenting to participate in this study. Variantyx Genomic Intelligence® is thanked for their assistance in the interpretation of the genetic analysis. Esther Eshkol is thanked for editorial assistance.
Funding
This study was funded by the Brain Center at Tel Aviv Sourasky Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All experiments were performed in accordance with relevant guidelines and regulations. The study protocols were reviewed and approved by the Institutional Review Board. Informed consent was obtained from all participants prior to genetic testing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hausman-Kedem, M., Ben-Sira, L., Kidron, D. et al. Deletion in COL4A2 is associated with a three-generation variable phenotype: from fetal to adult manifestations. Eur J Hum Genet 29, 1654–1662 (2021). https://doi.org/10.1038/s41431-021-00880-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-021-00880-3
This article is cited by
-
Identification of a novel intronic variant in COL4A2 gene associated with fetal severe cerebral encephalomalacia and subdural hemorrhage
BMC Medical Genomics (2024)
-
Genotyping arrays, population genetic studies and clinical implications
European Journal of Human Genetics (2021)