Abstract
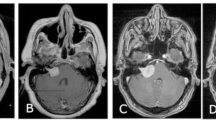
This study explores the natural history of vestibular, trigeminal and lower cranial nerve schwannomas (VS, TS, LCNS) in patients with Neurofibromatosis type 2 (NF2), to understand how pathogenic variants (PVs) of the NF2 gene affect tumour burden and growth rate, via a retrospective analysis of a UK NF2 centre database and imaging. VS, TS and LCNS location and size were measured in accordance with a standardised protocol. PVs were categorised in accordance with the UK NF2 Genetic Severity Score (GSS). 153 patients (age 5–82) had 458 schwannomas, of which 362 were previously untreated comprising: 204 VS, 93 TS, and 65 LCNS (IX, X, XI). 322 schwannomas had sequential imaging allowing growth rate analysis with a mean follow-up of 45 months. VS were universally present, and bilateral in 146/153 cases. 65% of tumours grew >2 mm during the study period at mean rate 2.0 mm/year. Significant association was found between increasing GSS and growth rate. TS occurred in 66/153 patients (bilateral in 27/153); 31% of tumours showed growth (mean 1.8 mm/yr). Significant increase in tumour prevalence was noted with increasing GSS. LCNS were found in 47/153 patients (bilateral in 19/153); 27% of tumours showed growth (mean 1.9 mm/yr). The trend for increased prevalence with increasing GSS did not reach significance. VS growth rate was significantly influenced by GSS and they were much more likely to grow than TS and LCNS. TS prevalence also correlated with increasing GSS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Lloyd SK, Evans DG. Neurofibromatosis type 2 (NF2): diagnosis and management. Handb Clin Neurol. 2013;115:957–67.
Evans DG, Bowers NL, Tobi S, Hartley C, Wallace A, King A, et al. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018;89:1215–9.
Evans DG, King AT, Tobi S, Wallace AJ, Perry M, Anup R, et al. Identifying the deficiencies of current diagnostic criteria for neurofibromatosis 2 using databases of 2777 individuals with molecular testing. Genet Med. 2019;21:1525–33.
Giovannini M, Robanus-Maandag E, Niwa-Kawakita M, Van der Valk M, Woodruff JM, Goutebroze L, et al. Schwann cell hyperplasia and tumours in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev. 1999;13:978–86.
Welling DB, Packer MD, Chang LS. Molecular studies of vestibular schwannomas: a review. Curr Opin Otolaryngol Head Neck Surg. 2007;15:341–6.
Mautner V, Lindenau M, Baser M, Hazim W, Tatagiba M, Hasse W, et al. The Neuroimaging and Clinical Spectrum of Neurofibromatosis 2. Neurosurgery. 1996;38:880–6.
Dirks MS, Butman JA, Kim HJ, Wu T, Morgan K, Tran AP, et al. Long-term natural history of neurofibromatosis Type 2—associated intracranial tumors. J Neurosurg. 2012;117:109–17.
Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16.
Fisher L, Doherty J, Lev M, Slattery W. Distribution of Nonvestibular Cranial Nerve Schwannomas in Neurofibromatosis 2. Otol Neurotol. 2007;28:1083–90.
Bakir M, Connor S, Thomas N, Barazi S. Trigeminal Schwannomas in Neurofibromatosis 2. J Neurol Surg B Skull Base. 2012;73 - A280 https://doi.org/10.1055/s-0032-1314195.
Moffat D, Kasbekar A, Axon P, Lloyd S. Growth Characteristics of Vestibular Schwannomas. Otol Neurotol. 2012;33:1053–8.
Stangerup S, Caye-Thomasen P, Tos M, Thomsen J. The Natural History of Vestibular Schwannoma. Otol Neurotol. 2006;27:547–52.
Mautner VF, Baser ME, Thakkar SD, Feigen UM, Friedman JM, Kluwe L. Vestibular schwannoma growth in patients with neurofibromatosis Type 2: a longitudinal study. J Neurosurg. 2002;96:223–8.
Harris GJ, Plotkin SR, MacCollin M, Bhat S, Urban T, Lev MH, et al. Three-Dimensional Volumetrics for Tracking Vestibular Schwannoma Growth in Neurofibromatosis Type 2. Neurosurgery. 2008;62:1314–9.
Picry A, Bonne NX, Ding J, Aboukais R, Lejeune JP, Baroncini M, et al. Long-term Growth Rate of Vestibular Schwannoma in Neurofibromatosis 2: A Volumetric Consideration. Laryngoscope. 2016;126:2358–62.
Slattery WH, Fisher LM, Iqbal Z, Oppenheimer M. Vestibular Schwannoma Growth Rates in Neurofibromatosis Type 2 Natural History Consortium Subjects. Otol Neurotol. 2004;25:811–7.
Ruttledge MH, Andermann AA, Phelan CM, Claudio JO, Han F, Chretien N, et al. Type of Mutation in the Neurofibromatosis Type 2 Gene (NF2) Frequently Determines Severity of Disease. Am J Hum Genet. 1996;59:331–42.
Selvanathan SK, Shenton A, Ferner R, Wallace AJ, Huson SM, Ramsden RT, et al. Further genotype-phenotype correlations in neurofibromatosis 2. Clin Genet. 2010;77:163–70.
Halliday D, Emmanouil B, Pretorius P, Mackeith S, Painter S, Tomkins H, et al. Genetic Severity Score predicts clinical phenotype in NF2. J Med Genet. 2017;54:657–64.
Gugel I, Grimm F, Teuber C, Kluwe L, Mautner VF, Tatagiba M, et al. Management of NF2-associated vestibular schwannomas in children and young adults: influence of surgery and clinical factors on tumor volume and growth rate. J Neurosurg Pediatr 2019;24:584–92.
O’Reilly BF, Mehanna H, Kishore A, Crowther JA. Growth rate of non-vestibular intracranial schwannomas. Clin Otolaryngol. 2004;29:94–97.
Fucci M, Buchman C, Brackman D, Berliner K. Acoustic Tumour Growth: Implications for Treatment Choices. Am J Otol. 1999;20:495–9.
Marshall A, Owen V, Nikolopoulos T, O’Donoghue G. Acoustic Schwannomas: Awareness Of Radiologic Error Will Reduce Unnecessary Treatment. Otol Neurotol. 2005;26:512–5.
DS S. 2x3 Contingency Table Exact Test Calculator. [Online];Date accessed May 2020. http://www.analyticscalculators.com.
Freeman G, Halton J. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951;1:141–9.
Neurofibromatosis Type 2 Service (All Ages). In: Highly Specialised Services 2018. NHS England. pp 52–3.
Lloyd SK, Evans DG. Neurofibromatosis type 2 service delivery in England. Neurochirurgie. 2018;64:375–80.
Baser ME, Makariou EV, Parry DM. Predictors of vestibular schwannoma growth in patients with neurofibromatosis Type 2. J Neurosurg. 2002;96:217–22.
Lewis D, Donofrio CA, O’Leary C, Li KL, Zhu X, Williams R, et al. The microenvironment in sporadic and neurofibromatosis type II-related vestibular schwannoma: the same tumor or different? A comparative imaging and neuropathology study. J Neurosurg. 2020; 134:1–11.
Equilibrium CoHa. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma [vestibular schwannoma]. Otolaryngol Head Neck Surg. 1995;113:179–80.
Mackeith S, Das T, Graves M, Patterson A, Donnelly N, Mannion R, et al. A comparison of semi-automated volumetric vs linear measurement of small vestibular schwannomas. Eur Arch Otorhinolaryngol. 2018;275:867–74.
Morris KA, Parry A, Pretorius PM. Comparing the sensitivity of linear and volumetric MRI measurements to detect changes in the size of vestibular schwannomas in patients with neurofibromatosis type 2 on bevacizumab treatment. Br J Radio. 2016;89:20160110.
Baser ME, Mautner VF, Parry DM, Evans DG. Methodological issues in longitudinal studies: vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42:903–6.
Author information
Authors and Affiliations
Contributions
Study conception and design: SRF, JW, OT, SKL, SAR, ES, CHW, ONP, RL, MJS, AJW, MK, DGE, and ATK. Data collection: DM, JW, OT, RS, CC, RL, and SRF. Data analysis: DM, JW, OT, CH, and SRF. Results interpretation: SRF, DM, JW, OT, SKL, SAR, ES, CHW, ONP, RL, MJS, AJW, MK, DGE, and ATK. Drafting the manuscript: DM, and JW. Manuscript revision and approval: all authors.
Corresponding author
Ethics declarations
Competing interests
DGE, SKL and MJS are supported by the all Manchester NIHR Biomedical Research Centre (IS-BRC-1215-20007). The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moualed, D., Wong, J., Thomas, O. et al. Prevalence and natural history of schwannomas in neurofibromatosis type 2 (NF2): the influence of pathogenic variants. Eur J Hum Genet 30, 458–464 (2022). https://doi.org/10.1038/s41431-021-01029-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-021-01029-y
This article is cited by
-
Long-read genome sequencing resolves the breakpoints of a chromosome 8;22 balanced translocation in NF2-related schwannomatosis
Scientific Reports (2025)
-
Prevalence, natural history and surgical outcome of spinal meningiomas in NF2-related schwannomatosis
Journal of Neuro-Oncology (2025)
-
NF2: An underestimated player in cancer metabolic reprogramming and tumor immunity
npj Precision Oncology (2024)
-
Imaging as an early biomarker to predict sensitivity to everolimus for progressive NF2-related vestibular schwannoma
Journal of Neuro-Oncology (2024)
-
Incidence and prevalence of neurofibromatosis type 1 and 2: a systematic review and meta-analysis
Orphanet Journal of Rare Diseases (2023)