Abstract
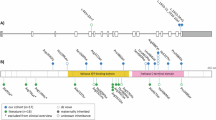
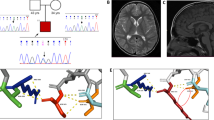
Pathogenic SOX11 variants have been associated with intellectual developmental disorder with microcephaly, and with or without ocular malformations or hypogonadotropic hypogonadism (HH) (IDDMOH, OMIM # 615866). In this article, we report seven new patients with de novo SOX11 variants. Five of the variants are missense, one nonsense, and one whole-gene deletion, most of them are novel variants. The main clinical features included neurodevelopmental delay (7/7) and intellectual disability (5/7), autism/attention deficit hyperactivity disorder (5/7), microcephaly (4/7), short stature (4/7), hypotonia (4/7), and clinodactyly of the 5th fingers (5/7). HH was confirmed in two female patients with primary amenorrhea, nonvisualized/prepubertal size of the uterus, and nonvisualized ovaries. Two of the male patients presented with micropenis, two had cryptorchidism, and one had decreased testicular size, which are suggestive findings of HH. This article contributes to the clinical characterization of patients with SOX11 variants and supports the role of this gene in HH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tsurusaki Y, Koshimizu E, Ohashi H, Phadke S, Kou I, Shiina M, et al. De novo SOX11 mutations cause Coffin-Siris syndrome. Nat Commun. 2014;5:1–7.
Hempel A, Pagnamenta AT, Blyth M, Mansour S, McConnell V, Kou I, et al. Deletions and de novo mutations of SOX11 are associated with a neurodevelopmental disorder with features of Coffin-Siris syndrome. J Med Genet. 2016;53:152–62.
Coffin GS, Siris E. Mental retardation with absent fifth fingernail and terminal phalanx. Am J Dis Child. 1970;119:433–9.
Vasko A, Drivas TG, Schrier Vergano SA. Genotype-Phenotype Correlations in 208 Individuals with Coffin-Siris Syndrome. Genes (Basel). 2021;12:937.
Cho CY, Tsai WY, Lee CT, Liu SY, Huang SY, Chien YH, et al. Clinical and molecular features of idiopathic hypogonadotropic hypogonadism in Taiwan: A single center experience. J Formos Med Assoc. 2022;121:218–26.
Al-Jawahiri R, Foroutan A, Kerkhof J, McConkey H, Levy M, Haghshenas S, et al. SOX11 variants cause a neurodevelopmental disorder with infrequent ocular malformations and hypogonadotropic hypogonadism and with distinct DNA methylation profile. Genet Med. 2022;24:1261–73.
Diel H, Ding C, Grehn F, Chronopoulos P, Bartsch O, Hoffmann EM.Correction to: First observation of secondary childhood glaucoma in Coffin-Siris syndrome: a case report and literature review. BMC Ophthalmol. 2021;21:1–5.
Hanker B, Gillessen-Kaesbach G, Hüning I, Lüdecke HJ, Wieczorek D. Maternal transmission of a mild Coffin–Siris syndrome phenotype caused by a SOX11 missense variant. Eur J Hum Genet. 2022;30:126–32.
Okamoto N, Ehara E, Tsurusaki Y, Miyake N, Matsumoto N. Coffin-Siris syndrome and cardiac anomaly with a novel SOX11 mutation. Congenit Anom (Kyoto). 2018;58:105–7.
Tsang SM, Oliemuller E, Howard BA. Regulatory roles for SOX11 in development, stem cells and cancer. Semin Cancer Biol. 2020;67:3–11.
Wang Y, Lin L, Lai H, Parada LF, Lei L. Transcription factor Sox11 is essential for both embryonic and adult neurogenesis. Developmental Dyn. 2013;242:638–53.
Zhao L, Arsenault M, Ng ET, Longmuss E, Chau TCY, Hartwig S, et al. SOX4 regulates gonad morphogenesis and promotes male germ cell differentiation in mice. Dev Biol. 2017;423:46–56.
Wakim V, Nair P, Delague V, Bizzari S, Al-Ali MTCC. SOX11-related syndrome: report on a new case and review. Clin Dysmorphol. 2021;30:44–9.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–303.
Sekiguchi F, Tsurusaki Y, Okamoto N, Teik KW, Mizuno S, Suzumura H, et al. Genetic abnormalities in a large cohort of Coffin–Siris syndrome patients. J Hum Genet. 2019;64:1173–86.
Australian Genomics Health Alliance Acute Care Flagship, Lunke S, Eggers S, Wilson M, Patel C, Barnett CP, et al. Baxend SZ. Feasibility of Ultra-Rapid Exome Sequencing in Critically Ill Infants and Children With Suspected Monogenic Conditions in the Australian Public Health Care System. JAMA Netw Open. 2020;323:2503–11.
Schrier Vergano S, Santen G, Wieczorek D, Wollnik B, Matsumoto N, Deardorff MA Coffin-Siris Syndrome. 2013 Apr 4 [updated 2021 Aug 12]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. PMID: 23556151.
Sreenivasan R, Gonen N, Sinclair A. SOX Genes and Their Role in Disorders of Sex Development. Sex Dev. 2022;16:80–91.
Barrionuevo F, Scherer G. SOX E genes: SOX9 and SOX8 in mammalian testis development. Int J Biochem Cell Biol. 2010;42:433–6.
Acknowledgements
The authors would like to thank the patients and their families for their participation in this study. Also, we want to thank Annabelle Tuttle, Erin Torti, and GeneDx for their support.
Funding
This study received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – Process number 2018/08890-9); Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Process #161749/2021-6 and #309782/2020-1), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) e Fundo de Apoio ao Ensino, à Pesquisa e à Extensão (FAEPEX – Unicamp).
Author information
Authors and Affiliations
Contributions
BSM, AMS, and TPV designed the study, wrote, and revised the article. AMS, VLGSL, AP, CFMS, EC, JCH, PJ, CS, ATMG, LM, AR, MS, HZE, JM, and SB evaluated the patients and collected clinical data. BSM, HFS, and SSS performed the in silico analysis. BSM and GRCC performed experimental analysis. TPV, NL, and RP performed the WES analysis. All authors revised the manuscript and approved the final version of this document.
Corresponding author
Ethics declarations
Competing interests
HZE and MS are employees of GeneDx, LLC. The other authors declare no competing interests.
Ethical approval
Written consent for clinical data collection was given by legal guardians. Ethical approval was obtained by the respective institutional research ethics board for each individual.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schincariol-Manhe, B., Campagnolo, É., Spineli-Silva, S. et al. Novel variants in the SOX11 gene: clinical description of seven new patients. Eur J Hum Genet 32, 1640–1646 (2024). https://doi.org/10.1038/s41431-024-01695-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-024-01695-8
This article is cited by
-
New guidelines for rare cancer syndromes
European Journal of Human Genetics (2024)