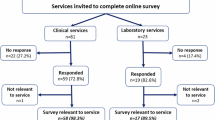
Abstract
Genetic testing of blood relatives of individuals at high risk of dominant conditions has significant preventive health benefits. However, cascade testing uptake is <50%. Research shows increased testing uptake when health professionals (HPs) contact at-risk relatives directly, with patient consent. Despite international support, this is not standard practice in Australia. We aimed to gather perspectives of genetic testing patients about direct-contact methods. Using an online survey, we surveyed Australian adults with genetic results of relevance for relatives, including patients who (i) self-categorised as being directly contacted by a clinical service, (ii) self-categorised as being referred by a HP, and (iii) received genetic results through a research study. Overall, 442 patients responded (clinical n = 363; research n = 79). Clinical patients self-categorised as 49.0% directly-contacted and 51.0% referred. Overall, the majority of patients had no privacy concerns about direct-contact methods (direct-contact 97%; referred 77%; research 76%). Less than 5% of the combined cohort (n = 19/442) reported significant concerns. The most prevalent concerns were the need for consent to provide HPs with relatives’ contact details, and a patient preference to notify relatives before HP contact. Other key findings include preferences about contact methods, including that most patients who received a letter from a genetics service preferred a letter with specific information about the familial genetic condition (n = 141/149; 94.6%) than one with general information about genetic risk. Our findings indicate Australian patients support HPs using direct-contact methods to assist with risk communication to relatives. Findings also identify concerns to be addressed in the design of direct-contact programs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Numerous data are made available via supplementary materials. Additional data can be made available on reasonable request.
References
Courtney E, Chok AK-L, Ting Ang ZL, Shaw T, Li S-T, Yuen J, et al. Impact of free cancer predisposition cascade genetic testing on uptake in Singapore. npj Genom Med. 2019;4:22.
Roberts MC, Dotson WD, DeVore CS, Bednar EM, Bowen DJ, Ganiats TG, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff. 2018;37:801–8.
George R, Kovak K, Cox SL. Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J Genet Couns. 2015;24:388–99.
Frey MK, Ahsan MD, Bergeron H, Lin J, Li X, Fowlkes RK, et al. Cascade testing for hereditary cancer syndromes: should we move toward direct relative contact? A systematic review and meta-analysis. J Clin Oncol. 2022;40:4129–43.
Forrest LE, Delatycki MB, Curnow L, Skene L, Aitken M. Genetic health professionals and the communication of genetic information in families: practice during and after a genetic consultation. Am J Med Genet Part A. 2010;152A:1458–66.
Healey E, Taylor N, Greening S, Wakefield CE, Warwick L, Williams R, et al. Quantifying family dissemination and identifying barriers to communication of risk information in Australian BRCA families. Genet Med. 2017;19:1323–31.
Srinivasan S, Won NY, Dotson WD, Wright ST, Roberts MC. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631–44.
Marleen van den Heuvel L, Stemkens D, van Zelst-Stams WAG, Willeboordse F, Christiaans I. How to inform at-risk relatives? Attitudes of 1379 Dutch patients, relatives, and members of the general population. J Genet Couns. 2020;29:786–99.
Rosén A, Krajc M, Ehrencrona H, Bajalica-Lagercrantz S. Public attitudes challenge clinical practice on genetic risk disclosure in favour of healthcare-provided direct dissemination to relatives. Eur J Hum Genet. 2024;32:6–7.
Gaff C, Hodgson J. A genetic counseling intervention to facilitate family communication about inherited conditions. J Genet Couns. 2014;23:814–23.
Hodgson JM, Metcalfe SA, Aitken M, Donath SM, Gaff CL, Winship IM, et al. Improving family communication after a new genetic diagnosis: a randomised controlled trial of a genetic counselling intervention. BMC Med Genet. 2014;15:33.
Sermijn E, Delesie L, Deschepper E, Pauwels I, Bonduelle M, Teugels E, et al. The impact of an interventional counselling procedure in families with a BRCA1/2 gene mutation: efficacy and safety. Fam Cancer. 2016;15:155–62.
Evans DGR, Binchy A, Shenton A, Hopwood P, Craufurd D. Comparison of proactive and usual approaches to offering predictive testing for BRCA1/2 mutations in unaffected relatives. Clin Genet. 2009;75:124–32.
van El CG, Baccolini V, Piko P, Cornel MC. Stakeholder views on active cascade screening for familial hypercholesterolemia. Healthcare. 2018;6:108.
Aktan-Collan K, Haukkala A, Pylvänäinen K, Järvinen HJ, Aaltonen LA, Peltomäki P, et al. Direct contact in inviting high-risk members of hereditary colon cancer families to genetic counselling and DNA testing. J Med Genet. 2007;44:732–8.
Megan CR, David Dotson W, Christopher SD, Erica MB, Deborah JB, Theodore GG, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff. 2018;37:801–8.
Marks D, Thorogood M, Neil SM. Cascade screening for familial hypercholesterolaemia: implications of a pilot study for national screening programmes. J Med Screen. 2006;13:156–9.
Henrikson NB, Blasi P, Figueroa Gray M, Tiffany BT, Scrol A, Ralston JD, et al. Patient and family preferences on health system-led direct contact for cascade screening. J Personal Med. 2021;11:538.
Dheensa S, Lucassen A, Fenwick A. Limitations and pitfalls of using family letters to communicate genetic risk: a qualitative study with patients and healthcare professionals. J Genet Counsel. 2018;27:689–701.
Öfverholm A, Karlsson P, Rosén A. The experience of receiving a letter from a cancer genetics clinic about risk for hereditary cancer. Eur J Hum Genet. 2024;32:539–44.
Tiller JM, Stott A, Finlay K, Boughtwood T, Madelli EO, Horton A, et al. Direct notification by health professionals of relatives at-risk of genetic conditions (with patient consent): views of the Australian public. Eur J Hum Genet. 2024;32:98–108.
Menko FH, van der Velden SL, Griffioen DN, Ait Moha D, Jeanson KN, Hogervorst FBL, et al. Does a proactive procedure lead to a higher uptake of predictive testing in families with a pathogenic BRCA1/BRCA2 variant? A family cancer clinic evaluation. J Genet Couns. 2024;33:615–22.
van den Heuvel LM, Hoedemaekers YM, Baas AF, Baars MJH, van Tintelen JP, Smets EMA, et al. A tailored approach to informing relatives at risk of inherited cardiac conditions: results of a randomised controlled trial. Eur J Hum Genet. 2022;30:203–10.
Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006;43:665–70.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JGA. metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021.
Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. Focus Health Profess Educ A Multi-Profess J. 2022;23:111–27.
Stott A, Madelli EQ, Boughtwood T, Nowak KJ, Otlowski M, Tiller J. Health professionals contacting patients’ relatives directly about genetic risk (with patient consent): current clinical practice and perspectives. Eur J Hum Genet. 2024; https://doi.org/10.1038/s41431-024-01730-8.
Nääs C, von Salomé J, Rosén A. Patients’ perceptions and practices of informing relatives: a qualitative study within a randomised trial on healthcare-assisted risk disclosure. Eur J Hum Genet. 2024;32:448–55.
Funding
JT is funded by a National Health and Medical Research Council (NHMRC) Investigator Grant (ID 2025900). This work is supported by Australian Genomics, funded by the Australian Government through the NHMRC (GNT2000001 and GNT2035846).
Author information
Authors and Affiliations
Contributions
JT conceived the project, designed the methodology and data collection instruments with EM, MM, NP, MJ, TB, KN and MO, supervised analysis of data and wrote the manuscript. MJ assisted with recruitment and data collection, and critically reviewed the manuscript. MM and NP assisted with recruitment and critically reviewed the manuscript. EM implemented the data collection forms, assisted with data collected and data analysis and contributed to the writing and review of the manuscript. KF assisted with data analysis and writing of portions of the manuscript. TB assisted with recruitment and data collection and critically reviewed the manuscript. KN and MO critically reviewed the manuscript. MO supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This project was granted approval by the Royal Children’s Hospital Human Research Ethics Committee on 7 November 2022, HREC reference number 79691, and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiller, J., Finlay, K., Madelli, E.O. et al. Patients’ perspectives regarding health professionals contacting their relatives about genetic risk directly (with patient consent). Eur J Hum Genet 33, 485–495 (2025). https://doi.org/10.1038/s41431-024-01764-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-024-01764-y
This article is cited by
-
What’s new in April’s EJHG?
European Journal of Human Genetics (2025)
-
Maximizing cascade genetic testing for disease prevention through direct notification of at-risk relatives
Nature Medicine (2025)
-
Health professionals contacting patients’ relatives directly about genetic risk (with patient consent): current clinical practice and perspectives
European Journal of Human Genetics (2025)