Abstract
Purpose
Primary objective—to investigate the effect of retinal vessel oxygen saturation (SO2) on macular oedema (ME) in retinitis pigmentosa (RP) patients. Secondary objective—to link the presence of ME to metabolic (oxygen saturation of retinal vessels, SO2), functional (multifocal electroretinography, mfERG) and structural (Spectral Domain Optical Coherent Tomography, SD-OCT) alterations in RP.
Design
Prospective, cross-sectional, non-interventional study.
Subjects
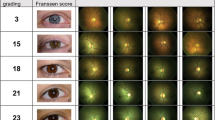
Patients with typical RP (N = 37) and controls (N = 19), who underwent retinal vessel Oximetry (RO), SD-OCT and mfERG, were included.
Methods
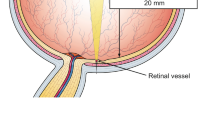
A computer-based program of the retinal vessel analyser unit (IMEDOS Systems UG, Jena, Germany) was used to measure SO2. We evaluated the mean SO2, in all major retinal arterioles (oxygen saturation in retinal arterioles, A-SO2, %) and venules (oxygen saturation in retinal venules, V-SO2, %). MfERG responses were averaged in zones (zone 1 (0–3°), zone 2 (3–8°) and zone 3 (8–15°)) and compared to corresponding areas of the OCT. The effect of ME on SO2 was evaluated dividing the RP in two subgroups: with clinical appearance of ME (ME-RP) and without it (no-ME-RP).
Main outcome measures
Parallel recording and juxtaposition of metabolic (SO2) to structural (OCT) and functional-(mfERG) measures. Mean ( ± SD) A-SO2 and V-SO2 were higher in no-ME-RP (96.77% (±6.31) and 59.93% (±7.76)) and even higher in the ME-RP (99.82% (±6.21) and 65.63% (±7.63)), compared to controls (93.15% (±3.76) and 53.77% (±3.70), p ≤ 0.006).
Results
The subgroup ME-RP differed significantly from the subgroup no-ME-RP by increased A-SO2 and V-SO2, p ≤ 0.026. The presence of ME confirmed a different relationship between the altered SO2 and the vessel diameters, against the functional and structural parameters.
Conclusion
Based on our results, the presence of macular oedema indicates a tendency toward greater alteration of the metabolic function in RP patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ammann F, Klein D, Franceschetti A. Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J Neurol Sci. 1965;2:183–96.
Puech B, Kostrubiec B, Hache JC, François P. Epidemiology and prevalence of hereditary retinal dystrophies in the Northern France. J Fr Ophtalmol. 1991;14:153–64.
Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40.
Hamel C. Cone rod dystrophies. Orphanet J Rare Dis. 2007;1:7.
Michaelides M, Hunt DM, Moore AT. The genetics of inherited macular dystrophies. J Med Genet. 2003;40:641–50.
Anderson B Jr, Saltzman HA. Retinal Oxygen utilisation measured by hyperbaric blackout. Arch Ophthalmol. 1964;72:792–5.
Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–57.
Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A, Miki K, Iwase T, Lu L, Campochiaro PA. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009;110:1028–37.
Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–64.
Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103:11300–5.
Todorova MG, Türksever C, Schorderet DF, Valmaggia C. Retinal vessel Oxygen Saturation in patients suffering from inherited diseases of the retina. Klin Monbl Augenheilkd. 2014;231:447–52.
Türksever C, Valmaggia C, Orgül S, Schorderet DF, Flammer J, Todorova MG. Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol. 2014;92:454–60.
Eysteinsson T, Hardarson SH, Bragason D, Stefánsson E. Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa. Acta Ophthalmol. 2014;92:449–53.
Ueda-Consolvo T, Fuchizawa C, Otsuka M, Nakagawa T, Hayashi A. Analysis of retinal vessels in eyes with retinitis pigmentosa by retinal oximeter. Acta Ophthalmol. 2015;93:e446–450.
Zong Y, Lin L, Yi C, Huang X, Fu Y, Dong Y, Qian X, Li Y, Gao Q. Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa at various ages. Graefes Arch Clin Exp Ophthalmol. 2016;254:243–52.
Bojinova RI, Türksever C, Schötzau A, Valmaggia C, Schorderet DF, Todorova MG. Reduced metabolic function and structural alterations in inherited retinal dystrophies: investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol. 2017;95:252–61.
Todorova MG, Türksever C, Schötzau A, Schorderet DF, Valmaggia C. Metabolic and functional changes in retinitis pigmentosa: comparing retinal vessel oximetry to full-field electroretinography, electrooculogram and multifocal electroretinography. Acta Ophthalmol. 2016;94:e231–41.
Lopez Torres LT, Türksever C, Schötzau A, Orgül S, Todorova MG. Peripapillary retinal vessel diameter correlates with mfERG alterations in retinitis pigmentosa. Acta Ophthalmol. 2015;93:e527–33.
Sandberg MA, Weigel-DiFranco C, Rosner B, Berson EL. The relationship between visual field size and electroretinogram amplitude in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1996;37:1693–8.
Gerth C, Wright T, Héon E, Westall CA. Assessment of central retinal function in patients with advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2007;48:1312–8.
Ma Y, Kawasaki R, Dobson LP, Ruddle JB, Kearns LS, Wong TY, Mackey DA. Quantitative analysis of retinal vessel attenuation in eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012;53:4306–14.
Nagy D, Schönfisch B, Zrenner E, Jägle H. Long-term follow-up of retinitis pigmentosa patients with multifocal electroretinography. Invest Ophthalmol Vis Sci. 2008;49:4664–71.
Wen Y, Klein M, Hood DC, Birch DG. Relationships among multifocal electroretinogram amplitude, visual field sensitivity, and SD-OCT receptor layer thicknesses in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012;53:833–40.
Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye. 2009;23:304–8.
Kim YJ, Joe SG, Lee DH, Lee JY, Kim JG, Yoon YH. Correlations between spectral-domain OCT measurements and visual acuity in cystoid macular edema associated with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54:1303–9.
Mitamura Y, Mitamura-Aizawa S, Katome T, Naito T, Hagiwara A, Kumagai K, Yamamoto S. Photoreceptor impairment and restoration on optical coherence tomographic image. J Ophthalmol. 2013;2013:518170
Konieczka K, Bojinova RI, Valmaggia C, Schorderet DF, Todorova MG, Medscape. Preserved functional and structural integrity of the papillomacular area correlates with better visual acuity in retinitis pigmentosa. Eye. 2016;30:1310–23.
Türksever C, Orgül S, Todorova MG. Reproducibility of retinal oximetry measurements in healthy and diseased retinas. Acta Ophthalmol. 2015;93:e439–45.
Schorderet DF, Iouranova A, Favez T, Tiab L, Escher P. IROme, a new high-throughput molecular tool for the diagnosis of inherited retinal dystrophies. Biomed Res Int. 2013;2013:198089.
Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–91.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115.
Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208.
Yu DY, Cringle SJ. Retinal degeneration and local oxygen metabolism. Exp Eye Res. 2005;80:745–51.
Padnick-Silver L, Kang Derwent JJ, Giuliano E, Narfström K, Linsenmeier RA. Retinal oxygenation and oxygen metabolism in Abyssinian cats with a hereditary retinal degeneration. Invest Ophthalmol Vis Sci. 2006;47:3639–3689.
Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41:3999–4006.
Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205.
Panfoli I, Calzia D, Bianchini P, Ravera S, Diaspro A, Candiano G, Bachi A, Monticone M, Aluigi MG, Barabino S, Calabria G, Rolando M, Tacchetti C, Morelli A, Pepe IM. Evidence for aerobic metabolism in retinal rod outer segment disks. Int J Biochem Cell Biol. 2009;41:2555–65.
Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–47.
Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–37.
Li ZY, Kljavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995;15:5429–38.
Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 2003;464:1–16.
Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287:1642–8.
Kostic C, Arsenijevic Y. Animal modelling for inherited central vision loss. J Pathol. 2016;238:300–10.
Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–65.
Fischer MD, Fleischhauer JC, Gillies MC, Sutter FK, Helbig H, Barthelmes D. A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:3617–21.
Sugita T, Kondo M, Piao CH, Ito Y, Terasaki H. Correlation between macular volume and focal macular electroretinogram in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2008;49:3551–8.
Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM, Sophie R, Mir TA, Scholl HP. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal. 2015;23:643–8.
Acknowledgements
Margarita G. Todorova was partially supported by unrestricted grant from OPOS (Stiftung Ostschweizerische Pleoptik- and Orthoptik-Schule) and by unrestricted grant from LHW (Liechtenstein Stiftung). We are extremely thankful to Nathaniel Jordan Lee; his technical support was invaluable for accomplishing our work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bojinova, R.I., Schorderet, D.F., Valmaggia, C. et al. Higher retinal vessel oxygen saturation: investigating its relationship with macular oedema in retinitis pigmentosa patients. Eye 32, 1209–1219 (2018). https://doi.org/10.1038/s41433-018-0043-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0043-1
This article is cited by
-
Full-field sensitivity threshold and the relation to the oxygen metabolic retinal function in retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
The impact of macular edema on microvascular and metabolic alterations in retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Optical coherence tomography angiography findings in patients undergoing transcorneal electrical stimulation for treating retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Metabolic monitoring of transcorneal electrical stimulation in retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)