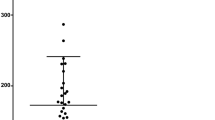Abstract
Purpose
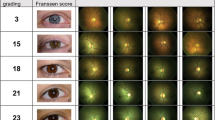
To study the retinal vessel oxygen saturation alterations in patients with autoimmune retinopathy (AIR) and patients with autoimmune retinopathy associated with retinitis pigmentosa (AIR-RP) in comparison with healthy controls and patients with isolated retinitis pigmentosa (RP).
Design
Prospective, cross-sectional, and non-interventional study.
Subjects
Retinal vessel oximetry (RO) was performed on a total of 139 eyes: six eyes suffering from AIR and four eyes with AIR-RP were compared to 59 healthy control eyes and to 70 eyes with RP.
Methods
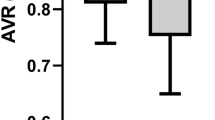
A computer-based program of the retinal vessel analyser unit (IMEDOS Systems UG, Jena, Germany) was used to evaluate retinal vessel oxygen saturation. The mean oxygen saturation in the first and second branch retinal arterioles (A-SO2) and venules (V-SO2) were measured and their difference (A-V SO2) was calculated. In addition, we measured the diameter of the retinal arterioles (D-A) and venules (D-V).
Main outcome measures
Oxygen metabolism is altered in patients with isolated AIR and AIR-RP.
Results
Both, AIR and AIR-RP groups, differed from healthy controls showing significantly higher V-SO2 values and significantly lower A-V SO2 values (p < 0.025). In addition, the AIR-RP group could be differentiated from eyes suffering from isolated RP by means of significantly higher V-SO2 values. Comparing retinal vessel diameters, both, the AIR and AIR-RP groups, presented with significant arterial (p = 0.05) and venular (p < 0.03) vessel attenuation than the healthy control group.
Conclusions
Based on our results, in analogy to patients suffering from RP, oxygen metabolism seems to be altered in AIR patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Schweitzer D, Thamm E, Hammer M, Kraft J. A new method for the measurement of oxygen saturation at the human ocular fundus. Int Ophthalmol. 2001;23:347–53.
Hammer M, Thamm E, Schweitzer D. A simple algorithm for in vivo ocular fundus oximetry compensating for non-haemoglobin absorption and scattering. Phys Med Biol. 2002;47:233–8.
Hardarson SH, Harris A, Karlsson RA, Halldorsson GH, Kagemann L, Rechtman E, et al. Automatic retinal oximetry. Invest Ophthalmol Vis Sci. 2006;47:5011–6.
Geirsdottir A, Palsson O, Hardarson SH, Olafsdottir OB, Kristjansdottir JV, Stefánsson E. Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci. 2012;13:5433–42.
Stefánsson E, Wolbarsht ML, Landers MB 3rd. In vivo O2 consumption in rhesus monkeys in light and dark. Exp Eye Res. 1983;37:251–6.
Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–57.
Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208.
Yu DY, Cringle SJ. Retinal degeneration and local oxygen metabolism. Exp Eye Res. 2005;80:745–51.
Türksever C, Valmaggia C, Orguel S, Schorderet DF, Todorova MG. Retinal vessel oxygen saturation in retinitis pigmentosa patients. In: EVER meeting2013 Nizza: E-abstract eTO23 Acta Ophthalmologica 2013;91:s252.
Todorova MG, Türksever C, Schorderet DF, Valmaggia C. Retinal vessel oxygen saturation in patients suffering from inherited diseases of the retina. Klin Monbl Augenheilkd. 2014;231:447–52.
Türksever C, Valmaggia C, Orgül S, Schorderet DF, Flammer J, Todorova MG. Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol. 2014;92:454–60.
Eysteinsson T, Hardarson SH, Bragason D, Stefánsson E. Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa. Acta Ophthalmol. 2014;92:449–53.
Ueda-Consolvo T, Fuchizawa C, Otsuka M, Nakagawa T, Hayashi A. Analysis of retinal vessels in eyes with retinitis pigmentosa by retinal oximeter. Acta Ophthalmol. 2015;93:446–50.
Todorova MG, Türksever C, Schötzau A, Schorderet DF, Valmaggia C. Metabolic and functional changes in retinitis pigmentosa: comparing retinal vessel oximetry to full-field electroretinography, electrooculogram and multifocal electroretinography. Acta Ophthalmol. 2016;94:231–41.
Bojinova RI, Türksever C, Schötzau A, Valmaggia C, Schorderet DF, Todorova MG. Reduced metabolic function and structural alterations in inherited retinal dystrophies: investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol. 2016;95:252–61.
Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81:606–13.
Grange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. Am J Ophthalmol. 2014;157:266–72.
Forooghian F, Macdonald IM, Heckenlively JR, Héon E, Gordon LK, Hooks JJ, et al. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2008;146:489–95.
Kondo M, Sanuki R, Ueno S, Nishizawa Y, Hashimoto N, Ohguro H, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS ONE. 2011;6:e19911.
Sen HN, Nussenblatt RB. Autoimmune retinopathies. In: Ryan, SJ, Schachat, AP, Wilkinson, CP, et al., editors. Retina 2013. 5th ed. United Kingdom: Elsevier; 2013: p. 1381–9.
Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48:12–38.
Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5–13.
Todorova MG, Palmowski-Wolfe AM, Leifert D, Prünte C, Meyer P. Unilateral nocturnal glare sensitivity and photopsias. Ophthalmologe. 2006;103:533–6.
Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127:390–7.
Sahel J, Bonnel S, Mrejen S, Paques M. Retinitis pigmentosa and other dystrophies. Dev Ophthalmol. 2010;47:160–7.
Fox AR, Gordon LK, Heckenlively JR, Davis JL, Goldstein DA, Lowder CY, et al. Consensus on the diagnosis and management of nonparaneoplastic autoimmune retinopathy using a modified delphi approach. Am J Ophthalmol. 2016;168:183–90.
Todorova MG, Bojinova RI, Valmaggia C, Schorderet DF. Prominent optic disc featured in inherited retinopathy. Klin Monbl Augenheilkd. 2017;234:577–83.
Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008;30:127–34.
Heckenlively JR, Solish AM, Chant SM, Meyers-Elliott RH. Autoimmunity in hereditary retinal degenerations. II. Clinical studies: antiretinal antibodies and fluorescein angiogram findings. Br J Ophthalmol. 1985;69:758–64.
Lopez Torres LT, Türksever C, Schötzau A, Orgül S, Todorova MG. Peripapillary retinal vessel diameter correlates with mfERG alterations in retinitis pigmentosa. Acta Ophthalmol. 2015;93:527–33.
Konieczka K, Bojinova RI, Valmaggia C, Schorderet DF, Todorova MG. Preserved functional and structural integrity of the papillomacular area correlates with better visual acuity in retinitis pigmentosa. Eye. 2016;30:1310–23.
Permeggiani F. Clinics, edidemiology and genetics of retinitis pigmentosa. Curr Genom. 2011;12:236–7.
Shahidi M, Wanek J, Blair NP, Little DM, Wu T. Retinal tissue oxygen tension imaging in the rat. Invest Ophthalmol Vis Sci. 2010;51:4766–70.
Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–47.
Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–55.
Cottet S, Schorderet DF. Mechanisms of apoptosis in retinitis pigmentosa. Curr Mol Med. 2009;9:375–83.
Hooks JJ, Tso MOM, Detrick B. Retinopathies associated with antiretinal antibodies. Clin Diagn Lab Immunol. 2001;8:853–8.
Keltner JL, Roth AM, Chang RS. Photoreceptor degeneration. Possible autoimmune disorder. Arch Ophthalmol. 1983;101:564–9.
Pirayesh A, Verbunt RJ, Kluin PM, Meinders AE, De Meijer PH. Myelodysplastic syndrome with vasculitic manifestations. J Intern Med. 1997;242:425–31.
Ju W, Park IA, Kim SH, Lee SE, Kim SC. Small cell carcinoma of the uterine corpus manifesting with visual dysfunction. Gynecol Oncol. 2005;99:504–6.
Acknowledgements
We thank Dr. Andy Schoetzau for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Waizel, M., Türksever, C. & Todorova, M.G. The effect of autoimmune retinopathy on retinal vessel oxygen saturation. Eye 32, 1455–1462 (2018). https://doi.org/10.1038/s41433-018-0122-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0122-3
This article is cited by
-
Retinal oxygen metabolic function in choroideremia and retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Full-field sensitivity threshold and the relation to the oxygen metabolic retinal function in retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
The impact of macular edema on microvascular and metabolic alterations in retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Optical coherence tomography angiography findings in patients undergoing transcorneal electrical stimulation for treating retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Metabolic monitoring of transcorneal electrical stimulation in retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)