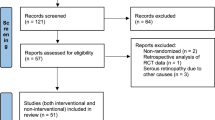
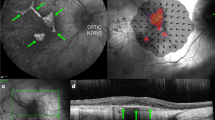
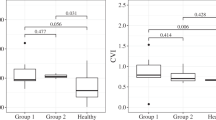
Abstract
Aims
Chronic central serous chorioretinopathy (CSCR) is poorly understood. Fluid accumulates in the subretinal space and retinal pigment epitheliopathy and neurosensory atrophy may develop. Permanent vision loss occurs in approximately one third of cases. There are no effective treatments for CSCR. Recent studies have shown the mineralocorticoid receptor antagonist, eplerenone, to be effective in resolving subretinal fluid and improving visual acuity. This trial aims to compare the safety and efficacy of eplerenone in patients with CSCR in a double-masked randomised placebo-controlled trial.
Methods
Patients are randomised 1:1 to receive eplerenone with usual care or placebo with usual care for 12 months; 25 mg per day for 1 week, then 50 mg per day up to 12 months (unless discontinued for safety or resolution of CSCR). Key eligibility criteria are: age 18–60 years, one eye with CSCR for ≥4 months duration, best-corrected visual acuity (BCVA) >53 and <86 letters and no previous treatment. The primary outcome is BCVA at 12 months. Secondary outcomes include resolution of subretinal fluid, development of macular atrophy, subfoveal choroidal thickness, changes in low luminance visual acuity, health-related quality of life and safety.
Conclusions
Recruitment is complete but was slower than expected. We maintained the eligibility criteria to ensure participants had ‘true’ CSCR and recruited additional centres. Effective distribution of the investigational medicinal product (IMP) was achieved by implementing a database to manage ordering and accountability of IMP packs. The results will provide adequately powered evidence to inform clinical decisions about using eplerenone to treat patients with CSCR.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22:19–24. PubMed PMID: 11884873.
Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond). 2010;24:1743–56. https://doi.org/10.1038/eye.2010.130. PubMed PMID: 20930852.
Schubert C, Pryds A, Zeng S, Xie Y, Freund KB, Spaide RF, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35:859–67. https://doi.org/10.1002/humu.22551. PubMed PMID: 24665005; PubMed Central PMCID: PMCPMC4215937.
Erikitola OC, Crosby-Nwaobi R, Lotery AJ, Sivaprasad S. Photodynamic therapy for central serous chorioretinopathy. Eye (Lond). 2014;28:944–57. Epub 2014/06/21. https://doi.org/10.1038/eye.2014.134. PubMed PMID: 24946843; PubMed Central PMCID: PMCPMC4135258.
Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756–65. https://doi.org/10.1016/j.ophtha.2008.04.014. PubMed PMID: 18538401.
Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841. https://doi.org/10.1002/14651858.CD011841.pub2. PubMed PMID: 26691378.
Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond). 2013;27:1339–46. Epub 2013/11/10. doi: 10.1038/eye.2013.236. PubMed PMID: 24202051; PubMed Central PMCID: PMCPMC3869506.
Zhao M, Celerier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672–9. https://doi.org/10.1172/Jci61427. PubMed PMID: WOS:000306044600037.
Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina-J Ret Vit Dis. 2013;33:2096–102. PubMed PMID: WOS:000330237900014.
Schwartz R, Habot-Wilner Z, Martinez MR, Nutman A, Goldenberg D, Cohen S, et al. Eplerenone for chronic central serous chorioretinopathy—a randomized controlled prospective study. Acta Ophthalmol. 2017. Epub 2017/06/28. https://doi.org/10.1111/aos.13491. PubMed PMID: 28653813.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65e5. https://doi.org/10.1016/j.ophtha.2008.10.018. PubMed PMID: 19118696.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. https://doi.org/10.1056/NEJMoa054481. PubMed PMID: 17021318.
Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114:1804–9. https://doi.org/10.1016/j.ophtha.2007.06.047. Epub 2007/10/03PubMed PMID: 17908590.
Ferris FL III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. Epub 1982/07/01. PubMed PMID: 7091289.
Hutton D, Smith N, Cappel-Porter H, Saw C, Rogers CA. Development of the “GeneSYS” database system to support trial data capture and conduct. 2013. In: Trials [Internet]. 29/11/2013. [66].
NetWORC UK. Network of ophthalmic reading centres UK. Available from: http://www.networcuk.com/.
Reeves BC, Wood JM, Hill AR. Reliability of high- and low-contrast letter charts. Ophthalmic Physiol Opt. 1993;13:17–26. Epub 1993/01/01. PubMed PMID: 8510943.
Becker R, Teichler G, Graf M. Reproducibility of visual acuity assessment in normal and low visual acuity. Strabismus. 2007;15:3–6. https://doi.org/10.1080/09273970601172435. Epub 2007/05/25PubMed PMID: 17523039.
Clinical efficacy and mechanistic evaluation of Eplerenone for central serous chorio-retinopathy the VICI study. 2016. Available from: https://njl-admin.nihr.ac.uk/document/download/2009989.
Acknowledgements
We would like to acknowledge the Macular Society and Fight for Sight for their feedback on the patient information leaflet and the DMSC and TSC for their oversight of the trial. The independent DMSC is formed of a Chairperson, two consultant ophthalmologists and one consultant cardiologist. The independent TSC is formed of a Chairperson, two consultant ophthalmologists, a consultant cardiologist, an ophthalmic statistician and patient and public involvement representatives; other TSC members with observer status represent the trial management team and the Sponsor.
Funding
This project is funded by the Efficacy and Mechanism Evaluation (EME) Programme, an MRC and NIHR partnership (ref: 13/94/15), with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D Division, Public Health Agency in Northern Ireland. The trial is sponsored by University Hospital Southampton NHS Foundation Trust, Southampton, UK. This study was designed and delivered in collaboration with the Clinical Trials and Evaluation Unit (CTEU), a UKCRC registered clinical trials unit which, as part of the Bristol Trials Centre, is in receipt of National Institute for Health Research CTU support funding.
Author contributions
AL conceived the trial; AL, SS, BCR, AC and CR obtained funding; AL, SS, BCR and CR designed the trial; AW, LE and LC managed the trial with input from AL, SS, BCR and CR; UC, SE and FB-C provided expert input; AC and AL set up the biobank; AW wrote the first draft of the manuscript. All authors reviewed the manuscript and amended/approved the final version
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
AW, LC, LE, CAR, AC, SE and BCR have no conflicts of interest. FB-C is an inventor on a patent protecting the use of MR antagonists in CSCR. SS has received research grants, travel grants, and speaker fees from Novartis, Bayer, Allergan, Roche, Heidelberg Engineering, and Optos. AL has received travel grants and speaker fees from Bayer and Roche.
Disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the MRC, NHS, NIHR or the Department of Health.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Willcox, A., Culliford, L., Ellis, L. et al. Clinical efficacy of eplerenone versus placebo for central serous chorioretinopathy: study protocol for the VICI randomised controlled trial. Eye 33, 295–303 (2019). https://doi.org/10.1038/s41433-018-0212-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0212-2