Abstract
Objectives
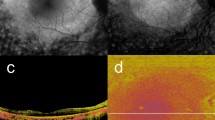
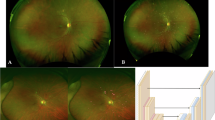
To document and characterise hyper- and hypo-reflective lesions, which we describe as ‘granular’ on short-wavelength autofluorescence (SW-AF) and near-infrared (NIR)-AF images in diabetic macular oedema (DMO).
Methods
Consecutive 103 eyes of 78 patients suffering from centre-involving DMO were reviewed retrospectively. Mosaics of hyper- and hypo-fluorescent dots on both SW-AF and NIR-AF signals were delineated and defined as granular lesions in the macula. We evaluated the association of such lesions with the logarithm of the minimum angle of resolution visual acuity (logMAR VA) and spectral-domain optical coherence tomography (SD-OCT) images.
Results
Diffuse mosaics of hyper- and hypo-fluorescent dots were delineated in 36 and 45 eyes on SW-AF and NIR-AF images, respectively, and both AF images defined granular lesions in 33 eyes. These lesions were delineated in both the fovea and extrafoveal areas on NIR-AF images but were limited to the parafoveal and perifoveal subfields on SW-AF images. There was a significant difference in logMAR VA between eyes with and without granular lesions (0.358 ± 0.269 vs. 0.185 ± 0.234; P = 0.001). Granular lesions were associated with the mosaic pattern on NIR-AF images (P < 0.001) but not with other parameters on SW-AF and NIR-AF images. The retinal thickness in the central subfield was greater in eyes with granular lesions (538.0 ± 163.6 μm vs. 448.8 ± 120.2 μm; P = 0.003). Granular lesions were associated with ELM disruption and hyper-reflective foci in the outer retinal layers (P = 0.004 and P = 0.037, respectively).
Conclusions
Granular lesions defined on both SW-AF and NIR-AF images were related to retinal oedema with photoreceptor damage and concomitant VA reduction in DMO.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39.
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–62.
Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–93.
Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:920713.
Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279–96.
Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724.
Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51:1051–61.
Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989;134:231–5.
Danis RP, Glassman AR, Aiello LP, Antoszyk AN, Beck RW, Browning DJ, et al. Diurnal variation in retinal thickening measurement by optical coherence tomography in center-involved diabetic macular edema. Arch Ophthalmol. 2006;124:1701–7.
Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 2013;33:740–7.
Murakami T, Tsujikawa A, Ohta M, Miyamoto K, Kita M, Watanabe D, et al. Photoreceptor status after resolved macular edema in branch retinal vein occlusion treated with tissue plasminogen activator. Am J Ophthalmol. 2007;143:171–3.
Sakamoto A, Nishijima K, Kita M, Oh H, Tsujikawa A, Yoshimura N. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1325–30.
Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, et al. Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53:1506–11.
Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31:1609–19.
Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–20.
Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol. 2012;153:710–7 e1.
Nishijima K, Murakami T, Hirashima T, Uji A, Akagi T, Horii T, et al. Hyperreflective foci in outer retina predictive of photoreceptor damage and poor vision after vitrectomy for diabetic macular edema. Retina. 2014;34:732–40.
Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718–29.
Delori FC, Fleckner MR, Goger DG, Weiter JJ, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:496–504.
Murakami T, Akimoto M, Ooto S, Suzuki T, Ikeda H, Kawagoe N, et al. Association between abnormal autofluorescence and photoreceptor disorganization in retinitis pigmentosa. Am J Ophthalmol. 2008;145:687–94.
Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409.
Bessho K, Gomi F, Harino S, Sawa M, Sayanagi K, Tsujikawa M, et al. Macular autofluorescence in eyes with cystoid macula edema, detected with 488 nm-excitation but not with 580 nm-excitation. Graefes Arch Clin Exp Ophthalmol. 2009;247:729–34.
Vujosevic S, Casciano M, Pilotto E, Boccassini B, Varano M, Midena E. Diabetic macular edema: fundus autofluorescence and functional correlations. Invest Ophthalmol Vis Sci. 2011;52:442–8.
Chung H, Park B, Shin HJ, Kim HC. Correlation of fundus autofluorescence with spectral-domain optical coherence tomography and vision in diabetic macular edema. Ophthalmology. 2012;119:1056–65.
Reznicek L, Dabov S, Haritoglou C, Kampik A, Kernt M, Neubauer AS. Green-light fundus autofluorescence in diabetic macular edema. Int J Ophthalmol. 2013;6:75–80.
Pece A, Isola V, Holz F, Milani P, Brancato R. Autofluorescence imaging of cystoid macular edema in diabetic retinopathy. Ophthalmologica. 2010;224:230–5.
Kang EC, Seo Y, Byeon SH. Diabetic retinal pigment epitheliopathy: fundus autofluorescence and spectral-domain optical coherence tomography findings. Graefes Arch Clin Exp Ophthalmol. 2016;254:1931–40.
Yoshitake S, Murakami T, Horii T, Uji A, Ogino K, Unoki N, et al. Qualitative and quantitative characteristics of near-infrared autofluorescence in diabetic macular edema. Ophthalmology. 2014;121:1036–44.
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 2011;151:310–7.
Keilhauer CN, Delori FC. Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest Ophthalmol Vis Sci. 2006;47:3556–64.
Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36.
Alasil T, Keane PA, Updike JF, Dustin L, Ouyang Y, Walsh AC, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology. 2010;117:2379–86.
Yoshitake S, Murakami T, Uji A, Unoki N, Dodo Y, Horii T, et al. Clinical relevance of quantified fundus autofluorescence in diabetic macular oedema. Eye (Lond). 2015;29:662–9.
Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600.
Rudolf M, Vogt SD, Curcio CA, Huisingh C, McGwin G Jr., Wagner A, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013;120:821–8.
Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–35.
Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81.
Sparrow JR, Yoon KD, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci. 2010;51:4351–7.
Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–42.
Ota M, Nishijima K, Sakamoto A, Murakami T, Takayama K, Horii T, et al. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002.
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Foveal cystoid spaces are associated with enlarged foveal avascular zone and microaneurysms in diabetic macular edema. Ophthalmology. 2011;118:359–67.
von Ruckmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol. 2002;133:780–6.
Murakami T, Uji A, Ogino K, Unoki N, Horii T, Yoshitake S, et al. Association between Perifoveal Hyperfluorescence and Serous Retinal Detachment in Diabetic Macular Edema. Ophthalmology. 2013;120:2596–603.
von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38:478–86.
Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003;110:392–9.
Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci USA. 2013;110:16586–91.
Paavo M, Lee W, Merriam J, Bearelly S, Tsang S, Chang S, et al. Intraretinal Correlates of Reticular Pseudodrusen Revealed by Autofluorescence and En Face OCT. Invest Ophthalmol Vis Sci. 2017;58:4769–77.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (17K11423).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yoshitake, S., Murakami, T., Uji, A. et al. Granular lesions of short-wavelength and near-infrared autofluorescence in diabetic macular oedema. Eye 33, 564–571 (2019). https://doi.org/10.1038/s41433-018-0256-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0256-3