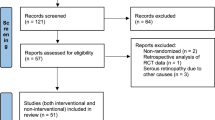
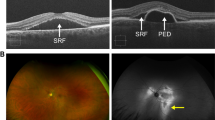
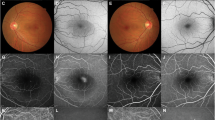
Abstract
Central serous chorioretinopathy (CSCR) is characterised by acute or chronic neurosensory detachments of the retina, usually in the posterior pole, with or without associated detachments of retinal pigment epithelium. Although the condition often resolves spontaneously, chronic and recurrent cases can lead to significant visual loss in the working population and it is thus increasingly recognised as an important public health issue. The uncertainty regarding the underlying cause of CSCR has led to a wide range of therapies being tried for this condition including photodynamic therapy, laser photocoagulation, anti-VEGF injections and a multitude of oral agents. This article aims to review the current evidence for oral agents that have been used for treatment of CSCR. A systematic literature search was conducted for articles published between 1980 to July 2018. A total of 73 articles were included. These studied the following oral medications: eplerenone, spironolactone, beta blockers, H. pylori agents, omeprazole, rifampicin, methotrexate, aspirin, acetazolamide, mifepristone, melatonin, finasteride, ketoconazole, antioxidants and curcumin phospholipid. Although none of the studies showed robust evidence of efficacy, the mineralocorticoid receptor antagonists, particularly eplerenone, appear to demonstrate the highest quality evidence for use in this condition. The review aims to give the reader an overview of the current available evidence for oral medications used in the treatment of CSCR in order to provide an evidence-based discussion with the patient and guide through possible options for treatment.
口服药物治疗中心性浆液性脉络膜视网膜病变的研究进展
摘要:
中心性浆液性脉络膜视网膜病变(Central serous chorioretinopathy,CSCR)常发生在视网膜的后极部, 以急性或慢性视网膜神经上皮脱离为主要特征, 可伴视网膜色素上皮层脱离。CSCR多见于青年及中年, 发病具有自限性, 但长期反复发作可以导致视力严重丧失。因此, CSCR已成为重要的公共健康问题。
CSCR的病因至今尚不明确, 临床上对其采取了多种治疗方案, 包括光动力治疗, 激光光凝术, 玻璃体腔注射抗VEGF药物以及口服药物治疗。本文对近年来关于口服药物治疗CSCR的研究进展作简要综述。
本文对1980年到2018年7月期间发表的关于口服药物治疗CSCR的文章进行了系统的文献检索, 共包含73篇文章, 研究包括下列口服药物: 依普利酮、螺内酯、β受体阻滞剂、抗幽门螺旋杆菌药物、奥美拉唑、利福平、甲氨蝶呤、阿司匹林、乙酰唑胺、米非司酮、褪黑素、非那雄胺、酮康唑、抗氧化剂和姜黄素磷脂。
目前针对各类口服药物治疗CSCR缺乏有效的临床研究, 但有较明确的证据显示盐皮质激素受体拮抗剂在CSCR的治疗中疗效显著, 尤其是依普立酮。
本文概述了目前口服药物治疗CSCR的研究进展, 可为患者提供循证证据, 并指导可行的临床治疗方案。
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gass JDM. Stereoscopic atlas of macular diseases. St Louis, MO: Mosby; 1997.
Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63:587.
Wong KH, Lau KP, Chhablani J, Tao Y, Li Q, Wong IY. Central serous chorioretinopathy: what we have learnt so far. Acta Ophthalmol (Copenh). 2016;94:321–5.
Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85–93.
Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010;149:307–15.e2.
Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011;31:119–26.
Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41:201–14.
Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980−2002. Ophthalmology. 2008;115:169–73.
Tsai DC, Chen SJ, Huang CC, Chou P, Chung CM, Huang PH, et al. Epidemiology of idiopathic central serous chorioretinopathy in Taiwan, 2001–2006: a population-based study. PLoS ONE. 2013;8:e66858.
Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111:244–9.
Schubert C, Pryds A, Zeng S, Xie Y, Freund KB, Spaide RF, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35:859–67.
Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47:431–48.
Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841.
Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672–9.
Van Dijk EH, Nijhoff MF, de Jong EK, Meijer OC, de Vries AP, Boon CJ. Central serous chorioretinopathy in primary hyperaldosteronism. Graefes Arch Clin Exp Ophthalmol. 2016;254:2033–42.
Schwartz R, Habot-Wilner Z, Martinez MR, Nutman A, Goldenberg D, Cohen S, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol (Copenh). 2017;95:e610–18.
Rahimy E, Pitcher JD 3rd, Hsu J, Adam MK, Shahlaee A, Samara WA, et al. A randomized double-blind placebo-control pilot study of eplerenone for the treatment of central serous chorioretinopathy (ECSELSIOR). Retina. 2018;38:962–69.
Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013;33:2096–102.
Gergely R, Kovács I, Schneider M, Resch M, Papp A, Récsán Z, et al. Mineralocorticoid receptor antagonist treatment in bilateral chronic central serous chorioretinopathy: a comparative study of exudative and nonexudative fellow eyes. Retina. 2017;37:1084–91.
Rajesh B, Agrawal H, Peguda HK, Chhablani J. Predictors of outcome during eplerenone therapy in chronic central serous chorioretinopathy: a prospective, open-label pilot clinical study. Ophthalmic Surg, Lasers Imaging Retin. 2018;49:479–86.
Breukink MB, den Hollander AI, Keunen JEE, Boon CJF, Hoyng CB. The use of eplerenone in therapy-resistant chronic central serous chorioretinopathy. Acta Ophthalmol. 2014;92:e488–90.
Cakir B, Fischer F, Ehlken C, Bühler A, Stahl A, Schlunck G, et al. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:2151–7.
Daruich A, Matet A, Dirani A, Gallice M, Nicholson L, Sivaprasad S. et al. Oral mineralocorticoid-receptor antagonists: real-life experience in clinical subtypes of nonresolving central serous chorioretinopathy with chronic epitheliopathy. Transl Vis Sci Technol. 2016;5:2.
Rübsam A, Thieme CE, Schlomberg J, Winterhalter S, Müller B, Joussen AM, et al. Therapy rationale for mineralocorticoid-receptor antagonists, acetazolamide and a switch of therapy in nonresponders in central serous chorioretinopathy. J Ocul Pharm Ther. 2017;33:141–8.
Kapoor KG, Wagner AL. Mineralocorticoid antagonists in the treatment of central serous chorioretinopathy: a comparative analysis. Ophthalmic Res. 2016;56:17–22.
Zucchiatti I, Sacconi R, Parravano MC, Costanzo E, Querques L, Montorio D, et al. Eplerenone versus observation in the treatment of acute central serous chorioretinopathy: a retrospective controlled study. Ophthalmol Ther. 2018;7:109–18.
Chin EK, Almeida DR, Roybal CN, Niles PI, Gehrs KM, Sohn EH, et al. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol. 2015;9:1449–56.
Ghadiali Q, Jung JJ, Yu S, Patel SN, Yannuzzi LA. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016;36:611–8.
Joshi NP, Adam MK, Samara WA, Shahlaee A, Rahimy E, Aderman CM, et al. Optical density of subretinal fluid as a predictive biomarker for eyes with chronic central serous retinopathy treated with eplerenone. J Vitreoretina Dis. 2018;2:6–11.
Leisser C, Hirnschall N, Hackl C, Plasenzotti P, Findl O. Eplerenone in patients with chronic recurring central serous chorioretinopathy. Eur J Ophthalmol. 2016;26:479–84.
Pociej-Marciak W, Karska-Basta I, Ozog-Baran J, Kubicka-Trzaska A, Filemonowicz-Skoczek A, Romanowska-Dixon B. The use of mineralocorticoid receptor antagonists in chronic central serous chorioretinopathy. Klin Ocz. 2016;118:48–54.
Salz DA, Pitcher JD, Hsu J, Regillo CD, Fineman MS, Elliott KS, et al. Oral eplerenone for treatment of chronic central serous chorioretinopathy: a case series. Ophthalmic Surg, Lasers Imaging Retin. 2015;46:439–44.
Sampo M, Soler V, Gascon P, Ho GW, Hoffart L, Denis D, et al. Eplerenone treatment in chronic central serous chorioretinopathy. J Fr d'Ophtalmol. 2016;39:535–42.
Singh RP, Sears J, Bedi R, Schachat A, Kaiser PK, Ehlers J. Oral eplerenone for the management of chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2014;55:1927.
Cioboata M, Alexandrescu C, Hopinca CA, Pienaru MC, Merticariu A, Schmitzer S. A new treatment approach-Eplerenone-in central serous chorioretinopathy—case report. J Med Life. 2016;9:92.
Gruszka A. Potential involvement of mineralocorticoid receptor activation in the pathogenesis of central serous chorioretinopathy: case report. Eur Rev Med Pharm Sci. 2013;17:1369–73.
Willcox A, Culliford L, Ellis L, Rogers CA, Cree A, Chakravarthy U, et al. Clinical efficacy of eplerenone versus placebo for central serous chorioretinopathy: study protocol for the VICI randomised controlled trial. Eye. 2019;33:295.
Clinical Trials.Gov—Photodynamic Therapy Versus Eplerenone: Treatment Trial for Chronic Central Serous Chorioretinopathy (SPECT). https://clinicaltrials.gov/ct2/show/NCT03079141. Accessed 26 Apr 2019.
Bousquet E, Beydoun T, Rothschild PR, Bergin C, Zhao M, Batista R, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015;35:2505–15.
Pichi F, Carrai P, Ciardella A, Behar-Cohen F, Nucci P. Comparison of two mineralcorticosteroids receptor antagonists for the treatment of central serous chorioretinopathy. Int Ophthalmol. 2017;37:1115–25.
Sun X, Shuai Y, Fang W, Li J, Ge W, Yuan S, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018;102:1060–5.
Chai Y, Liu RQ, Yi JL, Ye LH, Zou J, Jiang N, et al. Clinical research of fenofibrate and spironolactone for acute central serous chorioretinopathy. Int J Ophthalmol. 2016;9:1444.
Falavarjani KG, Amirsardari A, Habibi A, Eshaghi A, Bakhti S, Aghdam KA. Visual and anatomical outcomes of spironolactone therapy in patients with chronic central serous chorioretinopathy. J Ophthalmic Vis Res. 2017;12:281.
Herold TR, Prause K, Wolf A, Mayer WJ, Ulbig MW. Spironolactone in the treatment of central serous chorioretinopathy - a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252:1985–91.
Herold TR, Rist K, Priglinger SG, Ulbig MW, Wolf A. Long-term results and recurrence rates after spironolactone treatment in non-resolving central serous chorio-retinopathy (CSCR). Graefes Arch Clin Exp Ophthalmol. 2017;255:221–9.
Lee JY, Kim DY, Lee EK, Lee SY, Lee HJ, Jeong JH, et al. Comparison of short-term clinical outcomes between oral spironolactone and observation in acute central serous chorioretinopathy. J Korean Ophthalmol Soc. 2018;59:511–8.
Lee JH, Lee SC, Kim H, Lee CS. Comparison of short-term efficacy between oral spironolactone treatment and photodynamic therapy for the treatment of nonresolving central seroud chorioretinopathy. Retina. 2019;39:127–33.
Kim JY, Chae JB, Kim J, Kim DY. Mineralocorticoid receptor antagonist treatment for steroid-induced central serous chorioretinopathy patients with continuous systemic steroid treatment. J Ophthalmol. 2018;2018:4258763.
Maier M, Stumpfe S, Feucht N, Strobl P, Rath V, Lohmann CP. Mineralocorticoid receptor antagonists as treatment option for acute and chronic central serous chorioretinopathy. Ophthalmologe. 2014;111:173–80.
Ángel-Pereira D, Rocha-Cabrera P, Cordovés-Dorta L, Losada Castillo MJ, Blasco Alberto A, Abreu Reyes JA. Spironolactone, a therapeutic alternative in the treatment of diffuse retinal pigment epitheliopathy. Arch Soc Esp Oftalmol. 2016;91:599–603.
Kapoor KG, Sim J. Spironolactone for secondary central serous chorioretinopathy: a challenge−rechallenge case. Case Rep Ophthalmol. 2017;8:370–4.
Ryan EH, Pulido CM. Central serous chorioretinopathy treatment with spironolactone: a challenge-rechallenge case. Retin Cases Brief Rep. 2015;9:235–8.
Craft J. Eplerenone (Inspra), a new aldosterone antagonist for the treatment of systemic hypertension and heart failure. Bayl Univ Med Cent Proc. 2004;17:217–20.
Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, et al. Safety profile of mineralocorticoid receptor antagonists: spironolactone and eplerenone. Int J Cardiol. 2015;200:25–9.
Avci R, Deutman AF. Treatment of central serous choroidopathy with the beta receptor blocker metoprolol (preliminary results). Klin Monbl Augenheilkd. 1993;202:199–205.
Chatziralli I, Kabanarou SA, Parikakis E, Chatzirallis A, Xirou T, Mitropoulos P. Risk factors for central serous chorioretinopathy: multivariate approach in a case-control study. Curr Eye Res. 2017;17:1–5.
Chrapek O, Jirkova B, Kandrnal V, Rehak J, Sin M. Treatment of central serous chorioretinopathy with beta-blocker metipranolol. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:3–6.
Browning DJ. Nadolol in the treatment of central serous retinopathy. Am J Ophthalmol. 1993;116:770–1.
Kianersi F, Fesharaki F. Effects of propranolol in patients with central serous chorioretinopathy. J Res Med Sci. 2008;13:103–7.
Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: an evaluation by serial OCT. J Ocul Pharmacol Ther. 2006;22:145–9.
Fabianova J, Porubska M, Cepilova Z. Central serous chorioretinopathy–treatment with beta blockers. Ceska a Slov Oftalmol: Cas Ceske Oftalmol Spol a Slov Oftalmol Spol. 1998;54:401–4.
D'Elios MM, Andersen LP. Helicobacter pylori: inflammation, immunity, and vaccines. Helicobacter. 2007;12(Suppl 1):15–9.
Bohr URM, Annibale B, Franceschi F, Roccarina D, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection—other Helicobacters. Helicobacter. 2007;12(Suppl 1):45–53.
Feghhi M, Hajiani E, Khataminia GH. Incidence of Helicobacter pylori in central serous chorioretinopathy: a case control study. Jundishapur J Microbiol. 2008;1:15–19.
Mitchell HM. The epidemiology of Helicobacter pylori. Curr Top Microbiol Immunol. 1999;241:11–30.
Giusti C. Central serous chorioretinopathy: a new extragastric manifestation of Helicobacter pylori? Analysis of a clinical case. Clin Ter. 2001;152:393–7.
Mauget-Faÿsse M, Kodjikian L, Quaranta M, Ben Ezra D, Trepsat C, Mion F, et al. Rôle de l'Helicobacter pylori dans la choriorétinopathie séreuse centrale et l'épithéliopathie rétinienne diffuse. Résultats de la première étude prospective pilote. J Fr Ophtalmol. 2002;25:1021–5.
Asensio-Sánchez VM, Rodríguez-Delgado B, García-Herrero E, Cabo-Vaquera V, García-Loygorri C. Coriorretinopatía serosa central como manifestación extradigestiva de infección gástrica por Helicobacter pylori. Arch Soc Esp Oftalmol. 2008;83:177–82.
Ahnoux-Zabsonre A, Quaranta M, Mauget-Faÿsse M. Prevalence of Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy: a complementary study. J Fr Ophtalmol. 2004;27:1129–33.
Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses. 2004;63:524–7.
Borrelli M, D'Alessio AC, Menzione M, Villani A, Piccolo G, Montella F, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274–8.
Misiuk-Hojło M, Michałowska M, Turno-Krecicka A. Helicobacter pylori—a risk factor for the development of the central serous chorioretinopathy. Klin Ocz. 2009;111:30–2.
Roshani M, Davoodi NA, Seyyedmajidi MR, et al. Association of Helicobacter pylori with central serous chorioretinopathy in Iranian patients. Gastroenterol Hepatol Bed Bench. 2014;7:63–67.
Maeda S, Mentis AF. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2007;12((Suppl 1):10–4.
Whittle BJR, Morschl E, Pozsár J, Moran AP, László F. Helicobacter pylori lipopolysaccharide provokes iNOS-mediated acute systemic microvascular inflammatory responses in rat cardiac, hepatic, renal and pulmonary tissues. J Physiol Paris. 2001;95:257–9.
Giusti C, Mauget-Faÿsse M. Helicobacter pylori and idiopathic central serous chorioretinopathy. Swiss Med Wkly. 2004;134:395–8.
Casella AMB, Berbel RF, Bressanim GL, Malaguido MR, Cardillo JA. Helicobacter pylori as a potential target for the treatment of central serous chorioretinopathy. Clinics. 2012;67:1047–52.
Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99–103.
Dang Y, Mu Y, Zhao M, Li L, Guo Y, Zhu Y. The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients. Ther Clin Risk Manag. 2013;9:355–60.
Zavoloka O, Bezditko P, Lahorzhevska I, Zubkova D, Ilyina Y. Clinical efficiency of Helicobacter pylori eradication in the treatment of patients with acute central serous chorioretinopathy. Graefe's Arch Clin Exp Ophthalmol. 2016;254:1737–42.
Masayoshi S, Nozomi F, Yutaka K. Rifampicin as an oral angiogenesis inhibitor targeting hepatic cancers. Cancer Res. 2009;69:4760–8.
UpToDate. Rifampin. http://www.uptodate.com/contents/rifampin-rifampicin-drug-information?source=search_result&selectedTitle=1%7E150. Accessed 30 Nov 2016.
Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharm Toxicol. 1999;39:1–17.
Pikuleva IA. Cytochrome P450s and cholesterol homeostasis. Pharm Ther. 2006;112:761–73.
Epocrates. Rifampicin. https://online.epocrates.com/noFrame/showPage.do?method=analyze&MonographId=280&MultiBrandAlert=false. Accessed 3 Oct 2016.
PDR.net. Rifampicin. 2011. http://www.pdr.net/drugpages/concisemonograph.aspx?concise=564. Accessed 3 Oct 2016.
Ravage Z, Packo K. Rifampin for treatment of central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2011;52:2137.
Ravage ZB, Packo KH, Creticos CM, Merrill PT. Chronic central serous chorioretinopathy responsive to rifampin. Retin Cases Brief Rep. 2012;6:129–32.
Steinle NC, Gupta N, Yuan A, Singh RP. Oral rifampin utilisation for the treatment of chronic multifocal central serous retinopathy. Br J Ophthalmol. 2012;96:10–3.
Pouw AE, Olmos de Koo LC. Oral rifampin for central serous retinopathy: a strategic approach in three patients. Ophthalmic Surg Lasers Imaging Retin. 2015;46:98–102.
Shulman S, Goldenberg D, Schwartz R, Habot-Wilner Z, Barak A, Ehrlich N, et al. Oral Rifampin treatment for longstanding chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:15–22.
Mårde Arrhén Y, Nylén H, Lövgren-Sandblom A, Kanebratt KP, Wide K, Diczfalusy U. A comparison of 4β-hydroxycholesterol : cholesterol and 6β-hydroxycortisol: cortisol as markers of CYP3A4 induction. Br J Clin Pharm. 2013;75:1536–40.
Khan MS, Sameen M, Lodhi AA, Ahmed M, Ahmed N, Kamal M, et al. Effect of half adult dose of oral Rifampicin (300mg) in patients with idiopathic central serous chorioretinopathy. Pak J Med Sci. 2016;32:1158.
Venkatesh R, Agarwal M, Kantha M. Efficacy of oral rifampicin in chronic central serous chorioretinopathy. Ther Adv Ophthalmol. 2018;10:2515841418807130.
Nelson J, Saggau DD, Nielsen JS. Rifampin induced hepatotoxicity during treatment for chronic central serous chorioretinopathy. Retin Cases Brief Rep. 2014;8:70–2.
Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharm Rev. 2005;57:163–72.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36.
Munoz R, Shaked Y, Bertolini F, Emmenegger U, Man S, Kerbel RS. Anti-angiogenic treatment of breast cancer using metronomic low-dose chemotherapy. Breast. 2005;14:466–79.
Baggott JE, Morgan SL, Vaughn WH. Differences in methotrexate and 7-hydroxymethotrexate inhibition of folate dependent enzymes of purine nucleotide biosynthesis. Biochem J. 1994;300:627–9.
Kurup SK, Oliver A, Emanuelli A, Hau V, Callanan D. Low-dose methotrexate for the treatment of chronic central serous chorioretinopathy: a retrospective analysis. Retina. 2012;32:2096–101.
Abrishami M, Mousavi M, Hosseini SM, Norouzpour A. Treatment of chronic central serous chorioretinopathy with oral methotrexate. J Ocul Pharm Ther. 2015;31:468–75.
Yamada R, Yamada S, Ishii A, Tane S. Evaluation of tissue plasminogen activator and plasminogen activator inhibitor-1 in blood obtained from patients of idiopathic central serous chorioretinopathy. Nippon Ganka Gakkai Zasshi. 1993;97:955–60.
Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol. 1999;127:477–8.
Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: a pathogenetic model. Clin Ophthalmol. 2011;5:239–43.
Pecora JL. Ibuprofen in the treatment of central serous chorioretinopathy. Ann Ophthalmol. 1978;10:1481–3.
Caccavale A, Imparato M, Romanazzi F, Negri A, Porta A, Ferentini F. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009;73:435–7.
Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Low-dose aspirin as treatment for central serous chorioretinopathy. Clin Ophthalmol. 2010;4:899–903.
Sibilia J, Ravaud P, Marck G. Digestive and hemorrhage complications of low-dose aspirin. Presse Med. 2003;32(37 Pt 2):S17–S28.
Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190–5.
Tripathi RC, Fekrat S, Tripathi BJ, Ernest JT. A direct correlation of the resolution of pseudophakic cystoid macular edema with acetazolamide therapy. Ann Ophthalmol. 1991;23:127–9.
Wolfensberger TJ, Dmitriev AV, Govardovskii VI. Inhibition of membrane-bound carbonic anhydrase decreases subretinal pH and volume. Doc Ophthalmol. 1999;97:261–71.
Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97:387–97.
Wolfensberger TJ, Chiang RK, Takeuchi A, Marmor MF. Inhibition of membrane-bound carbonic anhydrase enhances subretinal fluid absorption and retinal adhesiveness. Graefes Arch Clin Exp Ophthalmol. 2000;238:76–80.
Steinsapir KD, Tripathi RC, Tripathi BJ, Ernest JT. Inhibition of ocular gamma glutamyl transpeptidase by acetazolamide [letter]. Exp Eye Res. 1992;55:179–81.
Geyer DR, Gragoudas ES. Central serous chorioretinopathy. In: Albert DM, Jakobiec FA, editors. Principles and practice of ophthalmology. Philadelphia, USA: WB Saunders; 1994. p. 818–25.
Pomykala M, Rubin P, Rubin JS. Recurrent central serous chorioretinopathy associated with retinitis pigmentosa treated with carbonic anhydrase inhibitors. Retin Cases Brief Rep. 2016;10:205–7.
Das D, Nigam E. Resolution of cystoid macular edema by topical dorzolamide in a case of central serous chorioretinopathy: a case report. Sci J Med & Vis Res Foun. 2017;35.
Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109:1723–5.
Jung-Testas I, Baulieu EE. Inhibition of glucocorticosteroid action in cultured L-929 mouse fibroblasts by RU 486, a new anti-glucocorticosteroid of high affinity for the glucocorticosteroid receptor. Exp Cell Res. 1983;147:177–82.
Shoupe D, Mishell DR Jr, Brenner PF, Spitz IM. Pregnancy termination with a high and medium dosage regimen of RU 486. Contraception. 1986;33:455–61.
Nielsen JS, Weinreb RN, Yannuzzi L, Jampol LM. Mifepristone treatment of chronic central serous chorioretinopathy. Retina. 2007;27:119–22.
Nielsen JS, Bachhawat A, Jampol LM. A case of chronic severe central serous chorioretinopathy responding to oral mifepristone: update. Retina. 2008;28:1363.
Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011;31:1928–36.
Goldberg RA, Heier JS. Short-term oral mifepristone for the treatment of central serous chorioretinopathy (STOMP CSC)—a randomized, placebo-controlled study. Invest Ophthalmol Vis Sci. 2018;59:782–782.
Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pr Neurol. 2008;4:436–47.
Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–6.
Gramajo AL, Marquez GE, Torres VE, Juárez CP, Rosenstein RE, Luna JD. Therapeutic benefit of melatonin in refractory central serous chorioretinopathy. Eye (Lond). 2015;29:1036–45.
Townsend KA, Marlowe KF. Relative safety and efficacy of finasteride for treatment of hirsutism. Ann Pharm. 2004;38:1070–3.
Goetzl MA, Holzbeierlein JM. Finasteride as a chemopreventive agent in prostate cancer: impact of the PCPT on urologic practice. Nat Clin Pr Urol. 2006;3:422–9.
Kaufman KD, Rotonda J, Shah AK, et al. Long-term treatment with finasteride 1 mg decreases the likelihood of developing further visible hair loss in men with androgenetic alopecia (male pattern hair loss). Eur J Dermatol. 2008;18:400–6.
Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB. Finasteride for chronic central serous chorioretinopathy. Retina. 2011;31:766–71.
Moisseiev E, Holmes AJ, Moshiri A, Morse LS. Finasteride is effective for the treatment of central serous chorioretinopathy. Eye (Lond). 2016;30:850–6.
Chou SC, Lin JD. Longterm effects of ketoconazole in the treatment of residual or recurrent Cushing’s disease. Endocr J. 2000;47:401–6.
Winquist EW, Laskey J, Crump M, Khamsi F, Shepherd FA. Ketoconazole in the management of paraneoplastic Cushing’s syndrome secondary to ectopic adrenocorticotropin production. J Clin Oncol. 1995;13:157–64.
Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007;27:943–6.
Golshahi A, Klingmüller D, Holz FG, Eter N. Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol. 2010;88:576–81.
Ratanasukon M, Bhurayanontachai P, Jirarattanasopa P. High-dose antioxidants for central serous chorioretinopathy; the randomized placebo-controlled study. BMC Ophthalmol. 2012;12:20.
Mazzolani F. Pilot study of oral administration of a curcumin-phospholipid formulation for treatment of central serous chorioretinopathy. Clin Ophthalmol. 2012;6:801–6.
Mazzolani F, Togni S. Oral administration of a curcumin-phospholipid delivery system for the treatment of central serous chorioretinopathy: a 12-month follow-up study. Clin Ophthalmol. 2013;7:939–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fusi-Rubiano, W., Saedon, H., Patel, V. et al. Oral medications for central serous chorioretinopathy: a literature review. Eye 34, 809–824 (2020). https://doi.org/10.1038/s41433-019-0568-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-019-0568-y
This article is cited by
-
Central serous chorioretinopathy: updates in the pathogenesis, diagnosis and therapeutic strategies
Eye and Vision (2023)
-
Comparing treatment outcomes in randomized controlled trials of central serous chorioretinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Efficacy and safety of the mineralocorticoid receptor antagonist treatment for central serous chorioretinopathy: a systematic review and meta-analysis
Eye (2021)