Abstract
Purpose
To analyse the intraocular pressure rise after intravitreal dexamethasone implant (Ozurdex) amongst different geographic populations.
Methods
The medical charts of 294 dexamethasone implants between February 2011 and 2017 were reviewed retrospectively. South Asian (India), White (Europe, US and Israel) Latino (Argentina and Brazil) patient data was included in the study. Ocular hypertension (OHT) was defined as intraocular pressure of >25 mmHg or an increase of at least 10 mmHg from baseline. The main indications for treatment were diabetic macular edema (ME) (65.6%), retinal vein occlusion (26.5%), uveitis (7.8%).
Results
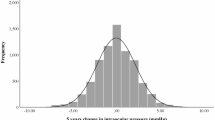
Amongst 294 intravitreal implants, ocular hypertension (>25 mmHg) was recorded in 0, 8 and 9.5% in White, Latino, and South Asian groups, respectively. However, IOP > 20 mmHg was recorded in 14%, 28% and 27% in White, Latino, and South Asian groups, respectively. Incidence of very high IOP (>35 mmHg) was lower in all geographical groups. It was 3% in Latino followed by 2% in South Asian group.
Conclusion
Latino and South Asian groups have higher IOP rise compared to White population. Most patients with elevated IOP fluctuate between 20–25 mmHg.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhang X, Clark AF, Yorio T. FK 506-binding protein 51 regulates nuclear transport of the glucocorticoid receptor beta and glucocorticoid responsiveness. Investig Ophthalmol Vis Sci. 2008;49:1037–47.
Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Investig Ophthalmol Vis Sci. 1990;31:2568–71.
Steely HT, Bowder SL, Julian MB, Miggans ST, et al. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Investig Ophthalmol Vis Sci. 1992;33:2242–50.
Rohen JW, Linner E, Witmer R. Electron microscopic studies on the trabecular meshwork in two cases of corticosteroid-glaucoma. Exp Eye Res. 1973;17:19–31.
Roll P, Benedikt O. Electron microscopic studies of the trabecular meshwork in corticosteroid glaucoma (in German). Klin Monatsbl Augenheilkd. 1979;174:421–8.
Singh IP, Ahmad SI, Yeh D, Challa P, et al. Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2004;138:286–7.
Sharma A, Patil AJ, Gupta N, Estrago-Franco MF, et al. Effects of triamcinolone acetonide on human trabecular meshwork cells in vitro. Indian J Ophthalmol. 2014;62:429–36.
Haller JA, Bandello F, Belfort R,Jr, Blumenkranz MS, Ozurdex GENEVA Study Group, Li J. et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology.2011;118:2453–60.
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, et al. Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14.
Bellocq D, Korobelnik JF, Burillon C, Voirin N, et al. Effectiveness and safety of dexamethasone implants for post-surgical macular oedema including Irvine-Gass syndrome: the EPISODIC study. Br J Ophthalmol. 2015;99:979–83.
Lowder C, Belfort R Jr, Lightman S, Foster CS, et al. Ozurdex HURON Study Group. dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–53.
Dot C, El Chehab H, Russo A, Agard E. Ocular hypertension after intravitreal steroid injections: clinical update as of 2015. J Fr Ophtalmol. 2015;38:656–64.
Malclès A, Dot C, Voirin N, Vié AL, et al. Safety of intravitreal dexamethasone implant (ozurdex): the SAFODEX study. Incidence and risk factors of ocular hypertension. Retina. 2017;37:1352–9.
Lam WC, Albiani DA, Yoganathan P, Chen JC, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–68.
Sarda V, Fajnkuchen F, Nghiem-Buffet S, et al. Early efficacy of dexamethasone implant (OZURDEX®) in diabetic macular edema: Real life study. J Fr Ophtalmol. 2017;40:408–13.
Pommier S, Meyer F, Guigou S, Barthelemy T, et al. Long-term real-life efficacy and safety of repeated Ozurdex® injections and factors associated with macular edema resolution after retinal vein occlusion: the REMIDO 2 study. Ophthalmologica 2016;236:186–92.
Jiménez-Gómez B, González-Montpetit M, Fonollosa Calduch A, Orive Bañuelos A, et al. Effects of ozurdex on intraocular pressure. A real life clinical practice study. Arch Soc Esp Oftalmol. 2015;90:421–5.
Reid GA, Sahota DS, Sarhan M. Observed complications from dexamethasone intravitreal implant for the treatment of macular edema in retinal vein occlusion over 3 treatment rounds. Retina 2015;35:1647–55.
Meyer LM, Schönfeld CL. Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a one-year follow-up. J Ocul Pharmacol Ther. 2013;29:560–5.
Theodoropoulou S, Ellabban AA, Johnston RL, Cilliers H, et al. Short-term safety of dexamethasone implant for treatment of macular edema due to retinal vein occlusion, in eyes with glaucoma or treated ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2017;255:725–32.
Mazzarella S, Mateo C, Freixes S, Burés-Jelstrup A, et al. Effect of intravitreal injection of dexamethasone 0.7 mg (Ozurdex®) on intraocular pressure in patients with macular edema. Ophthalmic Res. 2015;;54:143–9.
Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, et al. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Investig Ophthalmol Vis Sci. 2006; 47:4254–61.
Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol. 2011;129:914–20.
Jaffe GJ, Martin D, Callanan D, Pearson PA, et al. Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–7.
Chin EK, Almeida DRP, Velez G, Peraire M, et al. Ocular hypertension after intravitreal dexamethasone (Ozurdex) sustained-release implant. Retina.2017;37:1345–51.
Venediktos VKapetanakis, Michelle PYChan, Foster PJ, Cook DG, et al. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100:86–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ashish Sharma—consultant: Novartis India, Allergan Global, Intas India, Bayer India. BD Kuppermann—clinical research: Alcon, Alimera, Allegro, Allergan, Apellis, Clearside, Genentech, GSK, Ionis, jCyte, Novartis, Regeneron, ThromboGenics; consultant: Alimera, Allegro, Allergan, Cell Care, Dose, Eyedaptic, Galimedix, Genentech, Glaukos, Interface.
Biologics, jCyte, Novartis, Ophthotech, Regeneron, Revana, Theravance Biopharma. Francesco Bandello—consultant: Allergan, Bayer, Boehringer- Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Thrombogenics, Zeiss. Anat Loewenstein—consultant: Allergan, Novartis, Roche, Notal Vision, Fiorsightslabs, Beyeonics, Bayer Health Care.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Intraocular pressure (IOP) rise after Dexamethasone implant differs in different Geographic groups. South Asian and Latino groups have significantly higher incidence of IOP rise compared to Whites.
Rights and permissions
About this article
Cite this article
Sharma, A., Kuppermann, B.D., Bandello, F. et al. Intraocular pressure (IOP) after intravitreal dexamethasone implant (Ozurdex) amongst different geographic populations—GEODEX-IOP study. Eye 34, 1063–1068 (2020). https://doi.org/10.1038/s41433-019-0616-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-019-0616-7
This article is cited by
-
What have we learned from a decade treating patients with diabetic macular oedema with 0.19 mg fluocinolone acetonide intravitreal implant?
Eye (2025)
-
Gonioscopy-assisted transluminal trabeculotomy for intractable ocular hypertension after repeated intravitreal dexamethasone implant injections
International Ophthalmology (2025)
-
Comparing ranibizumab, dexamethasone implant, and combined therapy for macular edema secondary to branch retinal vein occlusion: a clinical trial
International Ophthalmology (2024)
-
Association of trabecular meshwork height with steroid-induced ocular hypertension
Scientific Reports (2023)
-
Efficacy and effectiveness of anti-VEGF or steroids monotherapy versus combination treatment for macular edema secondary to retinal vein occlusion: a systematic review and meta-analysis
BMC Ophthalmology (2022)