Abstract
Background
Many therapeutic options are available to glaucoma patients. One recent therapeutic option is minimally invasive glaucoma surgical (MIGS) devices. It is unclear how patients view different treatments and which patient-reported outcomes would be most relevant in patients with mild to moderate glaucoma. We developed a questionnaire for patients eligible for MIGS devices and a patient preference study to examine the value patients place on certain outcomes associated with glaucoma and its therapies.
Objectives
To summarize the progress to date.
Methods
Questionnaire development: We drafted the questionnaire items based on input from one physician and four patient focus groups, and a review of the literature. We tested item clarity with six cognitive interviews. These items were further refined.
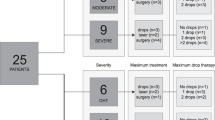
Patient preference study: We identified important benefit and risk outcomes qualitatively using semi-structured, one-on-one interviews with patients who were eligible for MIGS devices. We then prioritized these outcomes quantitatively using best-worst scaling methods.
Results
Questionnaire testing: Three concepts were deemed relevant for the questionnaire: functional limitations, symptoms, and psychosocial factors. We will evaluate the reliability and validity of the 52-item draft questionnaire in an upcoming field test.
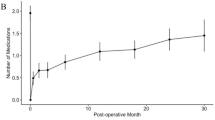
Patient preference study: We identified 13 outcomes that participants perceived as important. Outcomes with the largest relative importance weights were “adequate IOP control” and “drive a car during the day.”
Conclusions
Patients have the potential to steer clinical research towards outcomes that are important to them. Incorporating patients’ perspectives into the MIGS device development and evaluation process may expedite innovation and availability of these devices.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Glaukos iStent® Trabecular Micro-Bypass Stent (Models: GTS-100R, GTS-100L) and Inserter (GTS-100i) - P080030. 2012. www.accessdata.fda.gov/cdrh_docs/pdf8/P080030a.pdf; Accessed 2 Sep 2019.
FDA/AGS Workshop on supporting innovation for safe and effective minimally invasive glaucoma surgery. 2014. http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm382508.htm; Accessed 2 Sep 2019.
Premarket studies of implantable minimally invasive glaucoma surgical devices - guidance for industry and Food and Drug Administration staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/premarket-studies-implantable-minimally-invasive-glaucoma-surgical-migs-devices; Accessed 2 Sep 2019.
Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
Hunter NL, O’Callaghan KM, Califf RM. Engaging patients across the spectrum of medical product development: view from the US Food and Drug Administration. JAMA 2015;314:2499–500.
Food and Drug Administration. Guidance for industry patient preference information–voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling. http://bit.ly/2n5N9QE9 (2016) Accessed 12 Aug 2019.
Eydelman MB. The US Food and Drug Administration’s efforts to support ophthalmology clinical trials. JAMA Ophthalmol. 2014;132:1391–2.
Le JT, Viswanathan S, Tarver ME, Eydelman M, Li T. Assessment of the incorporation of patient-centric outcomes in studies of minimally invasive glaucoma surgical devices. JAMA Ophthalmol. 2016;134:1054–6.
Cui QN, Singh K, Spaeth GL. From the patient’s point of view, how should minimally invasive glaucoma surgeries be evaluated? Am J Ophthalmol. 2016;172:xii–xiv. https://doi.org/10.1016/j.ajo.2016.09.020
Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–9.
Tarver M, Eyldelman M. Incorporating patients’ perspectives. Glaucoma Today 2017. http://glaucomatoday.com/2017/04/incorporating-patients-perspectives; Accessed 12 Aug 2019.
Le JT, Mohanty K, Bicket AK, Tarver ME, Eydelman M, Li T. Identifying outcomes that are important to patients with ocular hypertension or primary open-angle glaucoma: a qualitative interview study. Ophthalmol Glaucoma. 2019;2:374–82.
Le JT, Bicket AK, Janssen EM, Grover D, Radhakrishnan S, Vold S, et al. Prioritizing outcome preferences in patients with open-angle glaucoma using best-worst scaling. Ophthalmol Glaucoma. 2019;2:367–73.
Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117.
Cheung KL, Wijnen BF, Hollin IL, et al. Using Best-Worst Scaling to investigate preferences in health care. Pharmacoeconomics 2016;34:1195–209.
Spaeth GL. Valid relevance in medical practice: the inadequacy of the linear model of health and disease: the Weisenfeld lecture. Investig Ophthalmol Vis Sci. 2011;52:6250–6.
Acknowledgements
We thank Beverly Weidmer for moderating focus groups and cognitive interviews, and Sylvia Paz for conducting additional cognitive interviews. We are appreciative of the input of the physician focus group members: Ron Fellman, Leslie Jones, Amanda Kiely, Shan Lin, Richard Parrish, Louis Pasquale, Douglas Rhee, Steven Sarkisian, Angela Tanna, Henry Trattler, Steve Vold, and George Spaeth (coauthor of this paper). We are grateful to Amanda Kiely Bicket, Davinder Grover, Sunita Radhakrishnan, and Steven Vold for assisting us with recruiting patients to participate in the Survey.
Meeting presentations
2016 Meeting of the American Glaucoma Society; Fort Lauderdale, FL, USA. 2017 Meeting of the American Glaucoma Society; San Diego, CA, USA. 2018 Meeting of the American Glaucoma Society; New York City, NY, USA. 2019 Meeting of the American Glaucoma Society; San Francisco, CA, USA.
Funding
This project was made possible by Grant Number U01FD005942 from the U.S. Food and Drug Administration (FDA), which supports the Johns Hopkins Center of Excellence in Regulatory Sciences and Innovation, and Grant Number U01FD004979/U01FD005978 from FDA, which supports the UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TL, RDH, QNC, and GS have no conflicts of interest to disclose. JTL was a doctoral student at the Johns Hopkins University when this work was conducted. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. ME and MET are employees of the U.S. Food and Drug Administration. KS has the following disclosures: Consultant: Alcon, Allergan, Glaukos, Ivantis, Santen, and Sight Sciences.
Ethics statement
Institutional Review Board (IRB) approval was obtained from the Johns Hopkins Bloomberg School of Public Health IRB (#7887) and the UCLA IRB (#16001107 AM00001, #16001107 AM00002, #18-001217). We obtained consent from all participants and we followed all participant data collection methods in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Le, J.T., Hays, R.D. et al. Patient-reported outcomes measures and patient preferences for minimally invasive glaucoma surgical devices. Eye 34, 205–210 (2020). https://doi.org/10.1038/s41433-019-0676-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-019-0676-8