Abstract
Introduction
Since 2007, the ocular 4:1 multiplex PCR assay in NHS Greater Glasgow and Clyde includes Chlamydia trachomatis (ocular chlamydia (OC)) testing. OC can be identified following routine ‘viral’ ophthalmic testing, including in asymptomatic patients. A published audit from 2008 identified only 25% of our OC patients attended and completed sexual health management, particularly when ophthalmologists initiated treatment. We subsequently created a shared care network between ophthalmology, virology and sexual health (including a designated sexual health advisor) to address these clinical issues.
Methods
A 10-year retrospective service review audit from January 2010 to December 2019 was performed to evaluate this approach.
Results
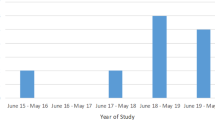
A total of 86 patients were identified (49 males (57%), median age 23 years (range 16–77)). Ophthalmologists initiated treatment for 37 patients (43%) prior to onward sexual health referral. Of this group, 5 (13.5%) received sub-optimal treatments, and 15 (40.5%) subsequently failed to attend sexual health services for partner notification. Of the 49 (57%) patients who attended sexual health, 25 (51%) had genital chlamydia co-infection, and 98% received adequate systemic treatment. All were offered full sexual health screening and 46 (93.9%) completed partner notification.
Conclusions
This shared care approach more than doubled the proportion of OC patients attending sexual health services over this 10-year period (previously 25%, now 57%). Ophthalmologists could defer treatment to sexual health for more effective OC management; however, challenges remain to address real-world issues of non-attendance, inadequate treatment and incomplete contact tracing. We recommend a multi-disciplinary approach to best manage OC cases identified following ophthalmic testing.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
World Health Organisation. Global health sector strategy on sexually transmitted infections, 2016-2021. 2016. https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/. Accessed 23 Mar 2020.
Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137.
Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. 2018. https://www.cdc.gov/std/stats17/default.htm. Accessed 23 Mar 2020.
Public Health England. Sexually transmitted infections and chlamydia screening in England, 2018. Health protection report. Volume 13, number 19. Public Health England; 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/806118/hpr1919_stis-ncsp_ann18.pdf. Accessed 23 Mar 2020.
Kalwij S, Macintosh M, Baraitser P. Screening and treatment of Chlamydia trachomatis infections. BMJ. 2010;340:c1915.
Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340:c1642.
Low N, Hocking J. The POPI trial: what does it mean for chlamydia control now? Sex Transm Infect. 2010;86:158–9.
Deschênes J, Seamone C, Baines M. The ocular manifestations of sexually transmitted diseases. Can J Ophthalmol. 1990;25:177–85.
Quirke M, Cullinane A. Recent trends in chlamydial and gonococcal conjunctivitis among neonates and adults in an Irish hospital. Int J Infect Dis. 2008;12:371–3.
Drummond SR, Diaper CJ. Chlamydial conjunctivitis presenting as pre septal cellulitis. Head Face Med. 2007;3:16–16.
Bersudsky V, Rehany U, Tendler Y, et al. Diagnosis of chlamydial infection by direct enzyme-linked immunoassay and polymerase chain reaction in patients with acute follicular conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 1999;237:617–20.
Elnifro EM, Cooper RJ, Klapper PE, et al. Multiplex polymerase chain reaction for diagnosis of viral and chlamydial keratoconjunctivitis. Investig Ophthalmol Vis Sci. 2000;41:1818–22.
Elnifro EM, Cooper RJ, Klapper PE, et al. Diagnosis of viral and chlamydial keratoconjunctivitis: which laboratory test? Br J Ophthalmol. 1999;83:622–7.
Kowalski RP, Thompson PP, Kinchington PR, et al. Evaluation of the SmartCycler II system for real-time detection of viruses and Chlamydia from ocular specimens. Arch Ophthalmol. 2006;124:1135–9.
Yip PP, Chan WH, Yip KT, et al. The use of polymerase chain reaction assay versus conventional methods in detecting neonatal chlamydial conjunctivitis. J Pediatr Ophthalmol Strabismus. 2008;45:234–9.
Mellman-Rubin TL, Kowalski RP, Uhrin M, et al. Incidence of adenoviral and chlamydial coinfection in acute follicular conjunctivitis. Am J Ophthalmol. 1995;119:652–4.
De Barbeyrac B, Benali L, Clerc M, et al. Chlamydia trachomatis infection in children: do not forget perinatal acquisition: a case report of a 7-year old girl, C. trachomatis infected, presumed sexually assaulted. J Forensic Leg Med. 2010;17:96–98.
Hammerschlag MR, Guillén CD. Medical and legal implications of testing for sexually transmitted infections in children. Clin Microbiol Rev. 2010;23:493–506. https://doi.org/10.1128/CMR.00024-09.
Lockington D, MacDonald R, King S, Weir C, Winter A, Aitken C. Multiplex PCR testing requires a robust multi-disciplinary strategy to effectively manage identified cases of chlamydial conjunctivitis. Scott Med J. 2013;58:77–82.
Winter AJ. ‘Managing patients differently: the Scottish National Sexual Health IT System (NaSH)’. In: Ilett R, Bigrigg A, editors. Transforming sexual health in Scotland: cultural, organisational and partnership approaches (e-book). Bentham Science Publishers; 2010. http://www.benthamscience.com/ebooks/9781608050659/index.htm.
McDaid L, Docherty S, Winter A. Occasional paper no. 24: review of the National Sexual Health IT System (NaSH) in Scotland: the potential for sexual health research. Glasgow: MRC/CSO Social and Public Health Sciences; 2013.
Garland SM, Malatt A, Tabrizi S, Grando D, Lees MI, Andrew JH, et al. Chlamydia trachomatis conjunctivitis. Preval Assoc Genit Trac Infect Med J Aust. 1995;162:363–6.
Althaus CL, Turner KM, Mercer CH, Auguste P, Roberts TE, Bell G, et al. Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling. Health Technol Assess. 2014;18:1–100. vii-viii.
Forbes G, Clutterbuck DJ. How many cases of chlamydial infection would we miss by not testing partners for infection? Int J STD Aids. 2009;20:267–8.
Chen YM, Hu FR, Hou YC. Effect of oral azithromycin in the treatment of chlamydial conjunctivitis. Eye. 2010;24:985–9.
Tobin JM, Bateman J, Banks B, Jeffs J. Clinical audit of the process of referral to genitourinary medicine of patients found to be chlamydia positive in a family planning service. Br J Fam Plann. 1999;24:160–3.
Estcourt CS, Gibbs J, Sutcliffe LJ, Gkatzidou V, Tickle L, Hone K, et al. The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: exploratory studies in people testing for Chlamydia trachomatis. Lancet Public Health. 2017;2:e182–90.
Estcourt CS, Sutcliffe LJ, Copas A, Mercer CH, Roberts TE, Jackson LJ, et al. Developing and testing accelerated partner therapy for partner notification for people with genital Chlamydia trachomatis diagnosed in primary care: a pilot randomised controlled trial. Sex Transm Infect. 2015;91:548–54.
Cameron ST, Glasier A, Muir A, Scott G, Johnstone A, Quarrell H, et al. Expedited partner therapy for Chlamydia trachomatis at the community pharmacy. BJOG. 2010;117:1074–9.
Willetts SJ, Cowper S, Cameron ST. An audit of a novel electronic messaging treatment service for Chlamydia trachomatis at a community pharmacy. Int J STD Aids. 2018;29:511–4.
Slutsker JS, Tsang LB, Schillinger JA. Do prescriptions for expedited partner therapy for chlamydia get filled? Findings from a multi-jurisdictional evaluation, United States, 2017-2019. Sex Transm Dis. 2020. https://doi.org/10.1097/OLQ.0000000000001163.
Gift TL, Kissinger P, Mohammed H, Leichliter JS, Hogben M, Golden MR. The cost and cost-effectiveness of expedited partner therapy compared with standard partner referral for the treatment of chlamydia or gonorrhea. Sex Transm Dis. 2011;38:1067–73.
Mmeje OO, Qin MJZ, Wetmore MMK, Kolenic MGE, Diniz MCP, Coleman JS. Breakdown in the expedited partner therapy treatment cascade: from reproductive healthcare provider to the pharmacist. Am J Obstet Gynecol. 2020;S0002-9378:30226–X. https://doi.org/10.1016/j.ajog.2020.02.038.
Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev. 2013;2013:CD002843.
Estcourt CS, Howarth AR, Copas A, et al. Accelerated partner therapy (APT) partner notification for people with Chlamydia trachomatis: protocol for the Limiting Undetected Sexually Transmitted infections to RedUce Morbidity (LUSTRUM) APT cross-over cluster randomised controlled trial. BMJ Open. 2020;10:e034806.
Acknowledgements
A version of this paper was an oral presentation at the Scottish Ophthalmological Club meeting, September 2019, in Stirling, Scotland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, M., Gishkori, S., Edington, M. et al. Ten-year review of a shared care approach in the management of ocular chlamydia trachomatis infections. Eye 35, 1614–1619 (2021). https://doi.org/10.1038/s41433-020-01128-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01128-y