Learning Objectives
Upon completion of this activity, participants will be able to:
-
1.
Assess demographic risk factors for radiation retinopathy after I-125 plaque brachytherapy for uveal melanoma.
-
2.
Analyze foveal avascular zone size in comparing eyes among patients treated for uveal melanoma.
-
3.
Evaluate capillary density in comparing eyes among patients treated for uveal melanoma.
Continuing Medical Education
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Springer Nature. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s). Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at www.medscape.org/journal/eye; (4) view/print certificate.
Credit hours
1.0
Release date:
Expiration date: November 23rd 2021
Post-test link: https://medscape.org/eye/posttest938103
Authors/Editors disclosure information
S.S. has disclosed the following relevant financial relationships: Served as an advisor or consultant for: Allergan, Inc.; Apellis; Bayer AG; Boehringer Ingelheim Pharmaceuticals, Inc.; Heidelberg Pharma GmbH; Novartis; Oculis; Optos; Oxurion; Roche. Served as a speaker or a member of a speakers bureau for: Allergan, Inc.; Bayer AG; Novartis Pharmaceuticals Corporation; Optos. Received grants for clinical research from: Allergan, Inc.; Bayer AG; Boehringer Ingelheim Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; Optos. T.E.d.C. and W.F.M. have disclosed no relevant financial relationships.
Journal CME author disclosure information
Charles P. Vega, MD, has disclosed the following relevant financial relationships: Served as an advisor or consultant for: GlaxoSmithKline.
Abstract
Objectives
To determine if commercial OCTA measurements can provide quantitative biomarkers for detection of radiation retinopathy (RR) s/p I-125 plaque brachytherapy in patients with uveal melanoma.
Methods
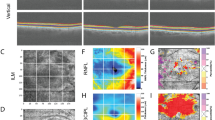
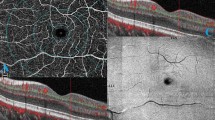
Retrospective review of 6 × 6 mm OCTA images of nonirradiated fellow eyes (group 1, 28 eyes), eyes without RR (group 2, 22 eyes), eyes with RR (group 3, 13 eyes). We used automated AngioVue AngioAnalytics OCTA software determinations of FAZ size, perimeter size, and 27 capillary density measurements (nine regions of each segmentation: full-thickness retina, superficial plexus, deep plexus).
Results
Average time since irradiation was 1.9 years in group 2, and 3.7 years in group 3. FAZ size was 1.2 mm in group 3 compared with 0.2 mm in group 1 and 0.3 mm in group 2 (both p < 0.001). Capillary density was statistically significantly reduced in group 3 compared with group 1 in all 27 regions. Group 2 had significantly decreased superficial plexus capillary density compared with group 1 in three regions. Group 3 had significantly reduced capillary density compared with group 2 in 6/27 (22%) regions. Linear regression showed a change in whole-scan density of −1.5 per year after irradiation in the full-thickness retina segmentation (p = 0.008).
Conclusion
Quantitative OCTA may aid in early detection of RR.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol. 2004;15:244–51.
Veverka KK, Abouchehade JE, Iexxi R, Pulido JS. Noninvasive grading of radiation retinopathy—the use of optical coherence tomography angiography. Retina. 2015;35:2400–10.
Shields CL, Say EAT, Samara WA, Khoo CTL, Mashayekhi A, Shields JA. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma—comparison of irradiated versus nonirradiated eyes in 65 patients. Retina. 2016;36:1493–505.
Say EAT, Samara WE, Khoo CTL, Magrath GN, Sharma P, Ferenczy S, et al. Parafoveal capillary density after plaque radiotherapy for choroidal melanoma—analysis of eyes without radiation maculopathy. Retina. 2016;36:1670–8.
Cennamo G, Breve MA, Velottia N. Evaluation of vascular changes with optical coherence tomography angiography after plaque radiotherapy of choroidal melanoma. Ophthalmic Res. 2018;60:238–42.
de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70.
Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, et al. Quantifying microvascular abnormalities with increasing severity of diabetic reinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:BIO307–15.
Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, et al. Select features of diabetic retinopathy on swept source optical coherence tomography angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol. 2016;134:644–50.
Adhi M, Filho M, Louzada R, Kuehlewein L, de Carlo TE, Baumal CR, et al. Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT486–94.
Onishi AC, Nesper PL, Roberts PK, Moharram GA, Chai H, Liu L, et al. Importance of considering the middle capillary plexus on OCT angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2167–76.
Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes. Acta Ophthalmol. 2017;95:e751–5.
Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostic tool. JAMA Ophthalmol. 2016;134:615–20.
Gunduz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation retinopathy following plaque radiotherapy for posterio uveal melanoma. Arch Ophtalmol. 1999;117:609–14.
Krema H, Xu W, Vasquez LM, Pavlin CJ, Simpson R. Factors predictive of radiation retinopathy post (125)Iodine brachytherapy for choroidal melanoma. Can J Ophthalmol. 2011;46:158–63.
Fenner BJ, Tan GSW, Tan ACS, Yeo IYS, Wong TY, Cheung GCM. Identification of imaging features that determine quality and repeatability of retinal capillary plexus density measurements in OCT angiography. Br J Ophthalmol. 2018;102:509–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
de Carlo, T.E., Mieler, W.F. Automated quantitative OCTA measurements of uveal melanoma-treated eyes with and without radiation retinopathy after I-125 plaque therapy and of nonirradiated fellow eyes. Eye 35, 769–776 (2021). https://doi.org/10.1038/s41433-020-01237-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01237-8
This article is cited by
-
Radiation Retinopathy
Current Ophthalmology Reports (2023)