Abstract
Background/Objective
Some clinicians may be forced to temporarily extend treatment intervals in neovascular age-related macular degeneration (nAMD) eyes with frequent retreatments to reduce the number of visits during the COVID-19 pandemic. To provide an indication of what these outcomes may be, we studied eyes with active lesions with unplanned treatment interval extensions before the pandemic occurred.
Methods
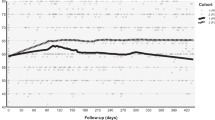
We compared eyes with active disease despite ≤6 weekly injections whose next injection was extended to ≥7 weeks and those whose intervals were not extended. We identified 1559 (16%) of 9602 eyes from the Fight Retinal Blindness! (FRB!) registry (2013 and 2018) that fit this criteria. Eyes were further stratified into four groups by the mean interval over the following 6 months: (1) ≤6 weeks (81%), (2) 7–9 weeks (9%), (3) 10–12 weeks (5%) and (4) >12 weeks (5%).
Results
There was a significant loss in VA in eyes extended to >12 weeks compared to the non-extended group (adjusted VA change, mean (95% CI): ≤6 weeks, 0.4 (−1.5 to 2.2), versus >12 weeks, −4.7 (−7.4 to −2.1), letters, p = 0.03 and a threefold increase in relative risk of losing ≥15 letters (absolute risk (14% versus 4%, p < 0.01)).
Conclusion
Mean VA remained stable for 6 months in eyes requiring frequent treatment despite retreatment interval extension up to 10–12 weeks. There was a significant short-term risk to vision when retreatment interval was extended beyond 12 weeks, hence extensions to this level should be considered cautiously. These data may be useful for physicians who are considering reducing visits to mitigate the risk of COVID-19.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74.
World Health Organisation. Coronavirus disease (COVID-2019) situation reports—situation report 51. Geneva, Switzerland: World Health Organisation; 2019.
Ahmed F, Zviedrite N, Uzicanin A. Effectiveness of workplace social distancing measures in reducing influenza transmission: a systematic review. BMC Public Health. 2018;18:518.
Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27:taaa020. https://doi.org/10.1093/jtm/taaa020.
Steffens I. A hundred days into the coronavirus disease (COVID-19) pandemic. Euro Surveill. 2020;25:2000550. https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000550.
Leung K, Wu JT, Liu D, Leung GM. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395:1382–93. https://doi.org/10.1016/S0140-6736(20)30746-7.
Cowling BJ, Ali ST, Ng TWY, Tsang TK, Li JCM, Fong MW, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. https://doi.org/10.1016/S2468-2667(20)30090-6.
Wee LE, Conceicao EP, Sim XYJ, Aung MK, Tan KY, Wong HM, et al. Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect. 2020;105:113–5. https://doi.org/10.1016/j.jhin.2020.04.016.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118:663–71.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. https://doi.org/10.1056/NEJMoa2002032.
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. https://doi.org/10.1001/jama.2020.2648.
Barthelmes D, Nguyen V, Daien V, Campain A, Walton R, Guymer R, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38:20–28.
Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126:723–34.
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37:1173–87.
Gillies MC, Walton R, Liong J, Arnold JJ, McAllister I, Morlet N, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the Fight Retinal Blindness! Project. Retina. 2014;34:188–95.
Foundation for Statistical Computing. R: a language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017.
Johnston RL, Carius HJ, Skelly A, Ferreira A, Milnes F, Mitchell P. A retrospective study of ranibizumab treatment regimens for neovascular age-related macular degeneration (nAMD) in Australia and the United Kingdom. Adv Ther. 2017;34:703–12.
Lanzetta P, Cruess AF, Cohen SY, Slakter JS, Katz T, Sowade O, et al. Predictors of visual outcomes in patients with neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor therapy: post hoc analysis of the VIEW studies. Acta Ophthalmol. 2018;96:e911–8.
Nguyen CL, Gillies MC, Nguyen V, Daien V, Cohn A, Banerjee G, et al. Characterization of poor visual outcomes of neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor agents. Ophthalmology. 2019;126:735–42.
Chong Teo KY, Saxena N, Gan A, Wong TY, Gillies MC, Chakravarthy U, et al. Detrimental Effect of Delayed Re-treatment of Active Disease on Outcomes in Neovascular Age-Related Macular Degeneration: The RAMPS Study. Ophthalmol Retina. 2020;4:871–80. https://doi.org/10.1016/j.oret.2020.03.017.
Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Quere S, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33:474–81.
Eldem B, Lai TYY, Ngah NF, Vote B, Yu HG, Fabre A, et al. An analysis of ranibizumab treatment and visual outcomes in real-world settings: the UNCOVER study. Graefes Arch Clin Exp Ophthalmol. 2018;256:963–73.
Holz FG, Bandello F, Gillies M, Mitchell P, Osborne A, Sheidow T, et al. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol. 2013;97:1161–7.
Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–59.
Invernizzi A, Nguyen V, Teo K, Barthelmes D, Fung A, Vincent A, et al. Five-year real-world outcomes of occult and classic choroidal neovascularization: data from the Fight Retinal Blindness! Project. Am J Ophthalmol. 2019;204:105–12.
Rakic JM, Leys A, Brie H, Denhaerynck K, Pacheco C, Vancayzeele S, et al. Real-world variability in ranibizumab treatment and associated clinical, quality of life, and safety outcomes over 24 months in patients with neovascular age-related macular degeneration: the HELIOS study. Clin Ophthalmol. 2013;7:1849–58.
Rao P, Lum F, Wood K, Salman C, Burugapalli B, Hall R, et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS Registry. Ophthalmology. 2018;125:522–8.
Essex RW, Nguyen V, Walton R, Arnold JJ, McAllister IL, Guymer RH, et al. Treatment patterns and visual outcomes during the maintenance phase of treat-and-extend therapy for age-related macular degeneration. Ophthalmology. 2016;123:2393–400.
Nguyen V, Vaze A, Fraser-Bell S, Arnold J, Essex RW, Barthelmes D, et al. Outcomes of suspending VEGF inhibitors for neovascular age-related macular degeneration when lesions have been inactive for 3 months. Opthamol Retina. 2019;3:623–8.
Chandra S, Flanagan D, Hingorani M, Lotery A, Sivaprasad S. COVID19 and ophthalmology: a brief summary of the literature. Eye (Lond). 2020;34:1200–2. https://doi.org/10.1038/s41433-020-0956-3.
Teo KYC, Chan RVP, Cheung CMG. Keeping our eyecare providers and patients safe during the COVID-19 pandemic. Eye (Lond). 2020;34:1161–2. https://doi.org/10.1038/s41433-020-0960-7.
Korobelnik JF, Loewenstein A, Eldem B, Joussen AM, Koh A, Lambrou GN, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:1149–56. https://doi.org/10.1007/s00417-020-04703-x.
Royal College of Ophthalmologists. Medical retinal management plans during COVID-19. Royal College of Ophthalmologists. https://www.rcophth.ac.uk/wp-content/uploads/2020/03/Medical-Retinal-Management-Plan-during-COVID-19-UPDATED-300320-1-3.pdf. Accessed 30 Mar 2020.
Maberley DMZ, Chaudhary V, Kertes P, Hooper P, Whelan J, Kherani A, et al. Additional considerations to help manage the anti-VEGF injection burden during the COVID-19 pandemic. Association of Canadian University Professors of Ophthalmology. https://www.cosprc.ca/resource/additional-considerations-to-help-manage-the-anti-vegf-injection-burden-during-the-covid-19-pandemic/. Accessed 4 Apr 2020.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48.
Acknowledgements
Fight Retinal Blindness! investigators: Auckland District Health Board, New Zealand (Dr D. Squirrell); Armadale Eye Clinic, Victoria (Dr A. Cohn); Lion’s Eye Institute, Western Australia (Dr F. Chen, Dr T. Isaac, Professor I. McAllister); Auckland Eye, New Zealand (Dr A. McGeorge); Bundaberg Eye Clinic, Queensland (Dr I. McLean); Cairns Eye Surgery, Queensland (Dr A. Field); Canberra Hospital, Australian Capital Territory (Dr C. Dayajeewa, Dr J. Wells); Care Foresight, New South Wales (Dr A. Dunlop); Caulfield Eye Clinic, Victoria (Dr C. Ng); Central Coast Eye Specialist, New South Wales (Dr S. Young); Centre for Eye Research Australia, Victoria (Professor R. Guymer); Coastwide Eye Surgery, New South Wales (Dr R. Ferrier); Crest Eye Associates, New Zealand (Dr J. Ah-Chan); Doncaster Eye Center, Victoria (Dr L. Chow); Dr Alex Amini’s Practice, Victoria (Dr A. Amini); Dr Clarks Practice, New South Wales (Dr G. Clark); Dr Nadia Wittles Practice, South Australia (Dr N. Wittles); Dr Phillip Windle, Queensland (Dr P. Windle); Eye Associates, New South Wales (Dr M. Gillies, Dr A. Hunt); Eye Surgeons Miranda, New South Wales (Dr A. Hunt); Eyemedics (Wayville), South Australia (Dr K. Billing, Dr J. Chen, Dr S. Lake, Dr J. Landers, Dr M. Perks, Dr R. Phillips, Dr N. Saha); Gladesville Eye Specialists, New South Wales (Dr S. Young); Hawthorn Eye Clinic, Victoria (Dr L. Chow); Hornsby Eye Specialists, New South Wales (Dr S. Lal); Les Manning, Queensland (Dr L. Manning); Marsden Eye Specialists, New South Wales (Dr J. Arnold); Midwest Ophthalmology, New South Wales (Dr K. Tang); Mona Vale Eye Centre, New South Wales (Dr C. Lim); Nepean Valley Eye Surgeons, New South Wales (Dr G. Banerjee); North Queensland Retina, Queensland (Dr I. Reddie); Port Macquarie Eye Centre, New South Wales (Dr J. Game); Retina & Macula Specialists (Miranda), New South Wales (Dr M. Chilov); Retina Associates, New South Wales (Dr S. Fraser-Bell, Dr A. Fung, Professor A. Hunyor, Dr C. Younan); Retina Consultants, New South Wales (Dr S. Young); Retina Specialists, New Zealand (Dr R. Barnes, Dr D. Sharp, Dr A. Vincent); Singapore National Eye Centre, Singapore (Dr G. Cheung); Specialist Eye Group, Victoria (Dr L. Chow, Dr A Cohn); Strathfield Retina Clinic, New South Wales (Dr C. Lim); Sydney Eye Hospital, New South Wales (Dr S. Fraser-Bell, Dr J. Wong); University Hospital Zurich, Switzerland (Dr D. Barthelmes); Victoria Parade Eye Consultants, Victoria (Dr M. Daniell, Professor R. Guymer, Dr A. Harper, Dr L. Lim, Dr J. ODay); Victorian Eye Surgeons, Victoria (Dr A. Cohn) and Visionary Eye Specialists, New South Wales (Dr C. Hooper).
Funding
Supported by a grant from the Royal Australian NZ College of Ophthalmologists Eye Foundation (2007–2009), a grant from the National Health and Medical Research Council, Australia, (NHMRC 2010–2012) and a grant from the Macula Disease Foundation, Australia. MCG is a Sydney Medical Foundation Fellow and is supported by an NHMRC practitioner fellowship. DB was supported by the Walter and Gertrud Siegenthaler Foundation Zurich, Switzerland, and the Swiss National Foundation. CMGC is supported grant by a grant from the National Medical Research Council (Open Fund Large Collaborative grant no: NMRC/LCG/0042018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MCG and DB are inventors of the software used to collect the data for this analysis, the copyright is owned by the University of Sydney.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teo, K.Y.C., Nguyen, V., Barthelmes, D. et al. Extended intervals for wet AMD patients with high retreatment needs: informing the risk during COVID-19, data from real-world evidence. Eye 35, 2793–2801 (2021). https://doi.org/10.1038/s41433-020-01315-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01315-x
This article is cited by
-
Treatment regimens for optimising outcomes in patients with neovascular age-related macular degeneration
Eye (2025)
-
Management of fibrosis in neovascular age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2025)
-
Reasons for Delayed Anti-VEGF Treatment During COVID-19 Lockdown and Clinical Impact in Neovascular Age-Related Macular Degeneration
Ophthalmology and Therapy (2023)
-
Are intravitreal injections essential during the COVID-19 pandemic? Global preferred practice patterns and practical recommendations
International Journal of Retina and Vitreous (2022)
-
Drop in well-being of ARMD patients under treatment with anti-VEGF injections during the COVID-19 pandemic
International Ophthalmology (2022)