Abstract
Purpose
To evaluate intravitreal conbercept injection for treatment of macular oedema secondary to central retinal vein occlusion (CRVO) in Chinese patients during 1-year follow-up in the real-world setting.
Methods
Twenty-seven eyes of 27 patients with macular oedema associated with CRVO were retrospectively reviewed. The eyes received monthly intravitreal conbercept injection (0.5 mg in 50 µl) for 3 months. From then on, the patients were followed up every month and received injection pro re nata (PRN) up to 12 months. The primary outcome measurements included changes of best-corrected visual acuity (BCVA) and central retinal thickness (CRT) from baseline to month 3 and month 12. Other outcome measurements included proportion of patients gaining ≥15 letters in BCVA at month 3 and 12, the mean number of injections and safety concerns.
Results
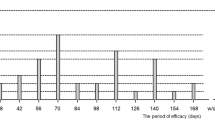
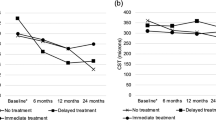
The mean BCVA gain from baseline was 12.7 ± 7.6 letters at month 3 and 14.8 ± 9.6 letters at month 12. The mean CRT reduction from baseline was 374.5 ± 280.7 μm at month 3 and 428.2 ± 241.3 μm at month 12. The proportion of patients who gained ≥15 letters in BCVA was 45.1% at month 3 and 52.9% at month 12. The mean number of injections was 7.6 ± 1.5. No severe local and systemic complications occurred following injection.
Conclusions
Intravitreal conbercept injection by three monthly loading doses followed by PRN treatment regimen was safe and efficacious for patients with macular oedema secondary to CRVO through 1-year follow-up.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9.
Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications: an update of the literature. Retina. 2013;33:901–10.
Yen YC, Weng SF, Chen HA, Lin YS. Risk of retinal vein occlusion in patients with systemic lupus erythematosus: a population-based cohort study. Br J Ophthalmol. 2013;97:1192–6.
Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, et al. The 10-year incidence and risk factors of retinal vein occlusion. Ophthalmology. 2013;120:803–8.
Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008;16:791–9.
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9.
Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121:1414–20.
Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009;93:452–6.
Wang Q, Li T, Wu Z, Wu Q, Ke X, Luo D, et al. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS One. 2013;8:e70544.
Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B, et al. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res 2009;26:204–10.
Yu DC, Lee JS, Yoo JY, Shin H, Deng H, Wei Y, et al. Soluble vascular endothelial growth factor decoy receptor FP3 exerts potent antiangiogenic effects. Mol Ther. 2012;20:938–47.
Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121:1740–7.
Liu K, Song Y, Xu G, Ye J, Wu Z, Liu X, et al. PHOENIX Study Group. Conbercept for treatment of neovascular age-related macular degeneration: results of the Randomized Phase 3 PHOENIX Study. Am J Ophthalmol. 2019;197:156–67.
Sun Z, Zhou H, Lin B, Jiao X, Luo Y, Zhang F, et al. Efficacy and safety of intravitreal conbercept injections in macular edema secondary to retinal vein occlusion. Retina. 2017;37:1723–30.
Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30:1046–50.
Ferrara N, Damico L, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–70.
Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400.
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–8.
Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, et al. GALILEO Study Group. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO Study. Ophthalmology. 2014;121:202–8.
Hykin P, Prevost AT, Vasconcelos JC, Murphy C, Kelly J, Ramu J, et al. LEAVO Study Group. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol. 2019;29. https://doi.org/10.1001/jamaophthalmol.2019.3305.
Campochiaro P, Hafiz G, Mir TA, Scott AW, Solomon S, Zimmer-Galler I, et al. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trial. Ophthalmology. 2015:122:1426–37.
Funding
This work was supported by Beijing Municipal Health System High-level Professionals training Program 2015 and Key research programme of Beijing Institute of Ophthalmology 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, Hy., Liu, Q., Li, Xx. et al. One-year efficacy of intravitreal conbercept injection for macular oedema secondary to central retinal vein occlusion in Chinese patients. Eye 34, 1459–1464 (2020). https://doi.org/10.1038/s41433-020-0827-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-0827-y
This article is cited by
-
Prognostic biomarkers in treatment-naïve central retinal vein occlusion with macular edema
European Journal of Medical Research (2025)
-
Changes in venous calibre during intra-vitreal therapy for central retinal vein occlusion
Eye (2021)