Abstract
Background
The Royal College of Ophthalmologists (RCOphth) recently produced new guidelines for the screening of hydroxychloroquine (HCQ) retinopathy. New imaging techniques have suggested an increased prevalence of retinopathy (7.5%) compared with previous studies (0.5%).
Methods
We collected prospective data from all patients referred to Sunderland Eye Infirmary, Sunderland for HCQ screening. Patients were screened according to RCOphth guidelines. In addition to retinal images, the data recorded included visual acuity, visual fields and multifocal electroretinography as appropriate, the patient’s age, diagnosis, weight, renal function and use of tamoxifen.
Results
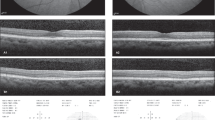
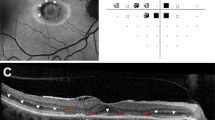
Of the 678 patients screened, 333 were categorised to be at risk (251 patients had been on HCQ >5 years, 117 had an estimated glomerular function rate <60 ml/min/1.73 m2, and 46 were on a dose >5 mg/kg/day). Eighty patients had multiple risk factors, 31 had been on doses of >5 mg/kg/day for >5 years. One hundred and sixty-eight of these patients have now been screened twice. The prevalence of HCQ retinopathy was 2/678 (0.3%) of all screened, 2/333 (0.6%) of patients at risk.
Conclusions
Our results show a far lower rate of retinopathy compared to the widely reported figure taken as standard by the RCOphth. This may be multifactorial: this prospective analysis has fewer patients taking higher doses of HCQ and shorter follow up, the comparison of serial images may highlight more cases and in addition, there are significant numbers of patients yet to be referred. Finally, the RCOphth’s diagnostic criteria is more exacting than that of the recent literature.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shee JC. Lupus erythematosus treated with chloroquine. Lancet. 1953;265:201–2.
Rogers J, Finn OA. Synthetic antimalarial drugs in chronic discoid lupus erythematosus and light eruptions. AMA Arch Derm Syphilol. 1954;70:61–6.
Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. JAMA. 1953;152:1428–9.
Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1958;2:478–80.
Ellsworth RJ, Zeller RW. Chloroquine induced retinal damage. AMA Arch Ophth. 1961;2:269–72.
Grupper C, Bregeat P, Juge P. Severe retinopathy during treatment of lupus erythematosus by synthetic antimalarials (2 cases). Bull de la Soc francaise de dermatologie et de syphiligraphie. 1963;70:824–32.
Lloyd LA, Hiltz JW. Ocular complications of chloroquine therapy. Can Med Assoc J. 1965;92:508–13.
Mackenzie AH, Scherbel AL. A decade of chloroquine maintenance therapy: rate of administration governs incidence of retinopathy. Arthritis Rheum. 1968;11:496 (Abstract).
Carr RE, Gouras P, Gunkel RD. Chloroquine retinopathy early detection by retinal threshold test. Arch Ophthalmol. 1966;75:171–8.
Percival SPB, Meanock I. Chloroquine: ophthalmological safety and clinical assessment in rheumatoid arthritis. Br Med J. 1968;3:579–84.
Finbloom DS, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985;12:692–4.
Morsman CDG, Livesey SJ, Richards IM, Jessop JD, Mills PV. Screening for hydroxychloroquine retinal toxicity: is it necessary? Eye. 1990;4:572–6.
Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62:775–84.
Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006;12:294–304.
Spalton DJ, Verdon Roes GM, Hughes GRV. Hydroxychloroquine, dosage parameters and retinopathy. Lupus. 1993;2:355–8.
Fielder A, Graham E, Jones S, Silman A, Tullo A. Royal College of Ophthalmologists guidelines: ocular toxicity and hydroxychloroquine. Eye. 1998;12:907–9.
Tang C, Godfrey T, Stawell R, Nikpour M. Hydroxychloroquine in lupus: emerging evidence supporting multiple beneficial effects. Intern Med J. 2012;42:968–78.
Abarientos C, Sperber K, Shapiro DL, Aronow WS, Chao CP, Ash JY. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin Drug Saf. 2011;10:705–14.
National Institute for Health and Care Excellence (NICE) Adalimumab, etanercept, infliximab, certolizumab, pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed TA375 published 26th January 2016. https://www.nice.org.uk/guidance/ta375/chapter/3-The-technologies.
Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453–60.
Public Health England Guidance Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. Updated 23 October 2015. https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme.
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, for the American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386–94.
Ledingham J, Gullick N, Irving K, Gorodkin R, Aris M, Burke J, on behalf of the BSR and BHPR Standards Guidelines and Audit Working Group, et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology. 2017;56:865–8.
Hydroxychloroquine and chloroquine retinopathy: recommendations on screening. RCO February 2018. https://www.rcophth.ac.uk/wpcontent/uploads/2018/07/Hydroxychloroquine-and-Chloroquine-Retinopathy-Screening-Guideline-Recommendations.pdf.
Marmor MF, Melles RB. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology. 2014;121:1257–62.
Garrity ST, Jung JY, Zambrowski O, Pichi F, Su D, Arya M, et al. Early hydroxychloroquine retinopathy: optical coherence tomography abnormalities preceding Humphrey visual field defects. Br J Ophthalmol. 2019;103:1600–4.
Browning DJ, Lee C. Relative sensitivity and specificity of 10-2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clin Ophthalmol (Auckl, NZ). 2014;8:1389–99.
Kunavisarut P, Chavengsaksongkram P, Rothova A, Pathanapitoon K. Screening for chloroquine maculopathy in populations with uncertain reliability in outcomes of automatic visual field testing. Indian J Ophthalmol. 2016;64:710–4.
“Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2018”. Office for National Statistics. 2019.
Yates M, Bechman K, Subesinghe S, Rutherford A, Russell M, Malaiya R, et al. Hydroxychloroquine use, risk of retinal toxicity and potential impact of screening in a large tertiary centre. Rheumatology. 2018;57:key075.188 (Abstract).
Hingorani M, Harcourt J Workforce census 2018 RCOphth. https://www.rcophth.ac.uk/publications/workforce-census-2018/.
Acknowledgements
We acknowledge the work of the photography department, the nursing staff, and clerical and secretarial staff at SEI who took on this work with no extra funding, equipment, time or staffing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gobbett, A., Kotagiri, A., Bracewell, C. et al. Two years’ experience of screening for hydroxychloroquine retinopathy. Eye 35, 1171–1177 (2021). https://doi.org/10.1038/s41433-020-1028-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-1028-4
This article is cited by
-
The incidence, monitoring coverage and clinical characteristics of hydroxychloroquine retinopathy in the United Kingdom
Eye (2024)
-
Outcomes of screening for hydroxychloroquine retinopathy at the Manchester Royal Eye Hospital: 2 years’ audit
Eye (2023)
-
Hydroxychloroquine screening in the UK-closing the gaps
Eye (2021)
-
Visual electrophysiology in the assessment of toxicity and deficiency states affecting the visual system
Eye (2021)
-
The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): executive summary
Eye (2021)