Abstract
Objectives
This study evaluated the 1-year treatment outcomes of bevacizumab for diabetic macular oedema (DMO) in routine clinical practice.
Methods
A retrospective analysis was performed on 298 eyes of 220 patients with DMO that received intra-vitreal bevacizumab between 1 September 2013 and 31 August 2018 that were tracked by a prospectively designed, web-based observational registry—the Fight Retinal Blindness! Registry.
Results
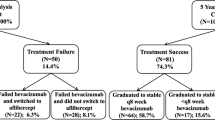
The mean visual acuity (95% confidence interval [CI]) at 1-year was 3 (2, 5) letters better than a mean (SD) of 68 (15) letters at study entry. Nearly a quarter of eyes achieved ≥20/40. Eyes presenting with better vision (≥20/40) tended to maintain that vision during the period of observation, whereas those presenting with worse vision (<20/40) gained a mean (95% CI) of 9 (5, 13) letters. A mean reduction in the macular thickness was observed over the study period with the central subfield improving by 29 µm (95% CI 17, 40) from a mean (SD) of 402 (109) µm at study entry. Eyes that completed 1 year of follow-up received a median (Q1, Q3) of 7 (4, 9) bevacizumab injections. Sixty-two eyes, ~20%, that started with bevacizumab changed to either another VEGF inhibitor or steroid (triamcinolone) during the period of observation. This did not lead to functional improvement for eyes changed to either ranibizumab or aflibercept despite a further reduction in macular thickness. An improvement in vision and reduction in macular thickness was noted in the 13 eyes that subsequently received triamcinolone. Approximately 10% of eyes dropped out over 12 months, even though their mean visual acuity had improved by seven letters from the initial visit.
Conclusions
Bevacizumab is an effective treatment for DMO in unselected populations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50.
Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8.
Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–84.
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47.
Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72.e5.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9.
Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018;6:Cd007419.
Parikh R, Ross JS, Sangaralingham LR, Adelman RA, Shah ND, Barkmeier AJ. Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and medicare advantage patients. Ophthalmology. 2017;124:352–8.
Gillies MC, Walton R, Liong J, Arnold JJ, McAllister I, Morlet N, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the fight retinal blindness! Project. Retina. 2014;34:188–95.
Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:48.
Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc (B). 2011;73:3–36.
Therneau Terry M. A package for survival analysis in S, version 2.38. https://CRAN.R-project.org/package=survival. Accessed 07 Mar 2020.
Australian Government Department of Health Therapeutic Goods Administration. Australian public assessment report for Ranibizumab. https://www.tga.gov.au/sites/default/files/auspar-ranibizumab-141014.doc. Accessed 20 Apr 2020.
Australian Government Department of Health Therapeutic Goods Administration. Australian public assessment report for Aflibercept. https://www.tga.gov.au/sites/default/files/auspar-aflibercept-150721.pdf. Accessed 20 Apr 2020.
Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retin. 2018;2:1179–87.
Holekamp NM, Campbell J, Almony A, Ingraham H, Marks S, Chandwani H, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91.
Bhandari S, Nguyen V, Fraser-Bell S, Mehta H, Viola F, Baudin F, et al. Ranibizumab or aflibercept for diabetic macular edema: comparison of 1-year outcomes from the Fight Retinal Blindness! Registry. Ophthalmology. 2020;127:608–15.
Biechl AC, Bhandari S, Nguyen V, Arnold JJ, Young S, Fraser-Bell S, et al. Changes in real-world treatment patterns for diabetic macular oedema from 2009–2019 and five-year outcomes: data from the Fight Retinal Blindness! Registry. Clin Exp Ophthalmol. 2020;48:802–12.
Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80.
Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. 2016;172:51–7.
Lukic M, Williams G, Shalchi Z, Sim D, Patel PJ, Keane PA, et al. Intravitreal aflibercept for diabetic macular oedema: Moorfields’ real-world 12-month visual acuity and anatomical outcomes. Eur J Ophthalmol. 2020;30:557–62.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77.e35.
Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36.
Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:257–69.
Weiss M, Sim DA, Herold T, Schumann RG, Liegl R, Kern C, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38:2293–300.
Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92.
Acknowledgements
Fight Retinal Blindness! Investigators: Auckland District Health Board, New Zealand (DS); Armadale Eye Clinic, Victoria (Dr A Cohn); Associate Professor Fred Chen’s Clinic, Western Australia (Professor F Chen); Australian Eye Specialists (Bacchus Marsh), Victoria (Dr N Jaross); Australian Eye Specialists (Wyndham), Victoria (Dr N Jaross); Bunbury and Busselton Eye Doctors, Western Australia (RB); Canberra Hospital, Australian Capital Territory (Dr R Essex, JMW); Central Coast Eye Specialist, New South Wales (Dr S Young); Eye Associates, New South Wales (MG); Eye Wide Bay, Queensland (Dr Z Louw); Gladesville Eye Specialists, New South Wales (Dr S Young); Marsden Eye Specialists, New South Wales (Dr J Arnold, Dr D Chan, TT); North Queensland Retina, Queensland (Dr I Reddie); Retina Associates, New South Wales (Dr S Fraser-Bell); Retina Specialists, New Zealand (RB); Specialist Eye Group, Victoria (Dr A Cohn); Sydney Eye Hospital, New South Wales (Dr S Fraser-Bell); Victorian Eye Surgeons, Victoria (Dr A Cohn).
Funding
The Fight Retinal Blindness! Project was supported by a grant from the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) Eye Foundation (2007–2009); a grant from the National Health and Medical Research Council, Australia (NHMRC 2010-2012); and a grant from the Macular Disease Foundation, Australia. MG is a Sydney Medical Foundation Fellow and is supported by an NHMRC practitioner fellowship. DB was supported by Walter Gertud Siegenthaler Foundation, Zurich, Switzerland and the Swiss National Foundation.
Author information
Authors and Affiliations
Contributions
Conception and design—SB, DS, VN, DB, MG. Analysis and Interpretation—SB, DS, VN, MG. Data collection—SB, DS, NW, JMW, TT, R Barnes, R Barry, MG. Overall responsibility—SB, DS, VN, NW, JMW, TT, R Barnes, R Barry, DB, MG.
Corresponding author
Ethics declarations
Conflict of interest
MG: grants from NHMRC, grants form RANZCO Eye Foundation, grants and others from Novartis, grants and other from Bayer and is an inventor of the software used to track real-world outcomes in this study. DB: received research grants from Novartis and Bayer and is an inventor of the software used to track real-world outcomes in this study. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhandari, S., Squirrell, D., Nguyen, V. et al. Bevacizumab for diabetic macular oedema: one-year treatment outcomes from the Fight Retinal Blindness! Registry. Eye 36, 594–602 (2022). https://doi.org/10.1038/s41433-021-01509-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01509-x