Abstract
Background
To assess the association between optical coherence tomography angiography (OCTA) retinal measurements and Alzheimer’s disease (AD).
Methods
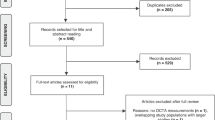
We searched MEDLINE and EMBASE from inception up to October 28th, 2020 for studies assessing the association between OCTA retinal measurements and AD. Estimates from eligible studies were meta-analysed and pooled standardized mean differences (SMDs) between AD patients and healthy participants with corresponding 95% confidence intervals (95% CI) were calculated, using the Hartung–Knapp/Sidik–Jonkman random-effects method. In addition, we quantified the minimum strength on the risk ratio scale (E value) required for an unmeasured confounder to nullify these associations.
Results
Ten eligible studies for our systematic review were identified through our search strategy. The pooled SMD between the retinal vessel density of AD patients and healthy participants in the whole superficial vascular plexus (SVP), parafoveal SVP and foveal avascular zone (FAZ) was −0.41 (95% CI: −0.69 to −0.13, p value = 0.01, I2 = 15%, seven studies), −0.51 (95% CI: −0.84 to −0.18, p value = 0.01, I2 = 40%, six studies), and 0.87 (95% CI: −0.03 to 1.76, p value = 0.05, I2 = 91%, seven studies), respectively. An unmeasured confounder would need to be associated with a 2.26-, 2.56- and 3.82-fold increase in the risk of AD and OCTA retinal measurements, in order for the pooled SMD estimate of vessel density in whole SVP, parafoveal SVP and FAZ, respectively, to be nullified.
Conclusions
In our study, whole and parafoveal SVP vessel density were inversely associated with AD. However, prospective longitudinal studies with larger sample sizes are needed to furtherly assess these associations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006239.
Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 2009;374:1821–30.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet 2006;368:387–403.
Lewis F. Estimation of future cases of dementia from those born in 2015. Consulting Report. Office of Health Economics, 2015.
Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10:135–42.
Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–48.
London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53.
Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013;33:1685–95.
Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–7.
Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology 2019;126:497–510.
Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT27–36.
Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–36.
Bulut M, Kurtuluş F, Gözkaya O, Erol MK, Cengiz A, Akıdan M, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102:233–7.
Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018;38:292–8.
Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol Retin. 2019;3:489–99.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12.
Wells GSB, Shea BJ, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/. Accessed 10 Nov 2019.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0147601.
IntHout J, Ioannidis JPA, Borm GF. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25.
Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79.
Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140–4.
Mathur MB, VanderWeele TJ. Sensitivity analysis for unmeasured confounding in meta-analyses. J Am Stat Assoc. 2020;115:163–72.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Chichester (UK): John Wiley & Sons. Cochrane, 2019. Available from: www.training.cochrane.org/handbook.
R Core Team. R: a language and environment for statistical computing. Austria: R Foundation for Statistical Computing V; 2018. https://www.R-project.org/.
Salobrar-García E, de Hoz R, Ramírez AI, López-Cuenca I, Rojas P, Vazirani R, et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS ONE. 2019;14:e0220535.
Lahme L, Esser EL, Mihailovic N, Schubert F, Lauermann J, Johnen A, et al. Evaluation of ocular perfusion in Alzheimer’s disease using optical coherence tomography angiography. J Alzheimers Dis. 2018;66:1745–52.
Querques G, Borrelli E, Sacconi R, De Vitis L, Leocani L, Santangelo R, et al. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9:63.
Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, Gebska-Toloczko M, Suwala K, Zabel K, et al. Comparison of retinal microvasculature in patients with Alzheimer’s disease and primary open-angle glaucoma by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60:3447–55.
den Haan J, van de Kreeke JA, van Berckel BN, Barkhof F, Teunissen CE, Scheltens P, et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement. 2019;11:383–91.
Li ZB, Lin ZJ, Li N, Yu H, Wu YL, Shen X. Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography. Int J Ophthalmol. 2021;14:860–8.
Wu J, Zhang X, Azhati G, Li T, Xu G, Liu F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol. 2020;98:e781–7.
Chua, J., Hu, Q., Ke, M. et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alz Res Therapy 2020;12:161. https://doi.org/10.1186/s13195-020-00724-0.
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the Optovue device. Dev Ophthalmol. 2016;56:6–12.
Rosenfeld PJ, Durbin MK, Roisman L, Zheng F, Miller A, Robbins G, et al. ZEISS Angioplex™ spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol. 2016;56:18–29.
Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Chichester (UK): John Wiley & Sons. Cochrane, 2020. Available from: www.training.cochrane.org/handbook.
Higgins JPT, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Chichester (UK): John Wiley & Sons. Cochrane, 2020. Available from: www.training.cochrane.org/handbook.
Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clin Inter Aging. 2007;2:347–59.
Klohs J. An integrated view on vascular dysfunction in Alzheimer’s disease. Neurodegenerative Dis. 2019;19:109–27.
Govindpani K, McNamara LG, Smith NR, Vinnakota C, Waldvogel HJ, Faull RL, et al. Vascular dysfunction in Alzheimer’s disease: a prelude to the pathological process or a consequence of it? J Clin Med. 2019;8:651.
Joyal J-S, Gantner ML, Smith LEH. Retinal energy demands control vascular supply of the retina in development and disease: the role of neuronal lipid and glucose metabolism. Prog Retin Eye Res. 2018;64:131–56.
Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. 2020;12:73–87.
Zhang J-F, Wiseman S, Valdés-Hernández MC, Doubal FN, Dhillon B, Wu Y-C, et al. The application of optical coherence tomography angiography in cerebral small vessel disease, ischemic stroke, and dementia: a systematic review. Front Neurol. 2020;11:1009.
Thal LJ, Kantarci K, Reiman EM, Klunk WE, Weiner MW, Zetterberg H, et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:6–15.
Author information
Authors and Affiliations
Contributions
Andreas K conceived and designed the presented study and performed the analysis. All authors wrote and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Katsimpris, A., Karamaounas, A., Sideri, A.M. et al. Optical coherence tomography angiography in Alzheimer’s disease: a systematic review and meta-analysis. Eye 36, 1419–1426 (2022). https://doi.org/10.1038/s41433-021-01648-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01648-1
This article is cited by
-
Early detection of dementia through retinal imaging and trustworthy AI
npj Digital Medicine (2024)
-
Identification of diabetic retinopathy classification using machine learning algorithms on clinical data and optical coherence tomography angiography
Eye (2024)
-
Enhancing foveal avascular zone analysis for Alzheimer’s diagnosis with AI segmentation and machine learning using multiple radiomic features
Scientific Reports (2024)
-
Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies
Neurology and Therapy (2023)