Abstract
Background
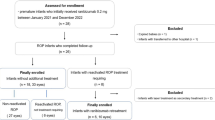
To assess reactivation after initial intravitreal injection of ranibizumab (IVR) for type 1 retinopathy of prematurity (ROP) or worse and the outcome following reinjection of ranibizumab for this reactivation.
Methods
This retrospective study was performed on infants screened for ROP between March 2013 and February 2020 in Mansoura University Children Hospital, Mansoura, Egypt. Infants treated with ranibizumab 0.25 mg/0.025 mL were identified for review of their clinical outcomes. Data of infants with reactivation and IVR re-injection were analysed.
Results
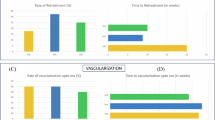
A total of 2318 infants were screened for ROP, 115 (5%) infants (216 eyes) with a mean gestational age of 30 ± 2.5 weeks and mean birth weight of 1290 ± 355.2 g received IVR at mean postmenstrual age (PMA) of 38 ± 3.1 weeks. All treated eyes demonstrated initial regression of ROP. However, ROP reactivation occurred in 5 (2.3%) eyes of 3 patients, at an average of 9.6 ± 2.9 weeks after treatment. None of these eyes had retinal detachment. A second dose IVR was administered and all five eyes showed regression with complete retinal vascularisation, at a mean PMA of 60 ± 5.1 weeks.
Conclusions
IVR is beneficial as an initial and subsequent treatment for type 1 ROP or APROP. A long-term follow-up until complete retinal vascularisation is recommended to avoid disease reactivation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82.
Kwinta P, Bik-Multanowski M, Mitkowska Z, Tomasik T, Pietrzyk JJ. The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1467–75.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–9
McNamara JA, Tasman W, Brown GC, Federman JL. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology. 1991;98:576–80.
Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–50.
Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl J Med. 2011;364:603–15.
Chen SN, Lian I, Hwang YC, ChenYH, Chang YC, Lee KH, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between Ranibizumab and Bevacizumab. Retina. 2015;35:667–74.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179–82.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855–9.
Wong RK, Hubschman S, Tsui I. Reactivation of retinopathy of prematurity after ranibizumab treatment. Retina. 2015;35:675–80.
Mintz-Hittner HA, Geloneck MM, Chuang AZ. Clinical Management of Recurrent Retinopathy of Prematurity after Intravitreal Bevacizumab Monotherapy. Ophthalmology. 2016;123:1845–55.
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Lemley CA, Han DP. An age-based method for planning sclerotomy placement during pediatric vitrectomy: a 12-year experience. Trans Am Ophthalmol Soc. 2007;105:86–91.
American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus & American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95.
Wang SD, Zhang GM, Shenzhen Screening for Retinopathy of Prematurity Cooperative Group. Laser therapy versus intravitreal injection of anti-VEGF agents in monotherapy of ROP: a Meta-analysis. Int J Ophthalmol. 2020;13:806–15.
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551–9.
Wu WC, Shih CP, Lien R, Wang NK, Chen YP, Chao AN, et al. Serum Vascular Endothelial Growth Factor After Bevacizumab Or Ranibizumab Treatment For Retinopathy Of Prematurity. Retina. 2017;37:694–701.
Huang Q, Zhang Q, Xu Y, Ji X, Fei P, Peng J, et al. Asymmetric Outcomes of Type 1 Retinopathy of Prematurity after Bilateral Intravitreal Ranibizumab Treatment. J Ophthalmol. 2017;2017:1741386.
Kimyon S, Mete A. Comparison of Bevacizumab and Ranibizumab in the Treatment of Type 1 Retinopathy of Prematurity Affecting Zone 1. Ophthalmologica. 2018;240:99–105.
Ling KP, Liao PJ, Wang NK, Chao AN, Chen KJ, Chen TL, et al. Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina. 2020;40:1793–803.
Chan J, Lam C, Kwok M, Wong R, Lee G, Lau W, et al. Risk of recurrence of retinopathy of prematurity after initial intravitreal ranibizumab therapy. Sci Rep. 2016;6:27082.
Erol MK, Coban DT, Sari ES, Bilgin AB, Dogan B, Ozdemir O, et al. Comparison of intravitreal ranibizumab and bevacizumab treatment for retinopathy of prematurity. Arq Bras Oftalmol. 2015;78:340–3.
Arámbulo O, Dib G, Iturralde J, Brito M, Fortes Filho JB. Analysis of the Recurrence of Plus Disease after Intravitreal Ranibizumab as a Primary Monotherapy for Severe Retinopathy of Prematurity. Ophthalmol Retin. 2018;2:858–63.
Lyu J, Zhang Q, Chen CL, Xu Y, Ji XD, Li JK, et al. Recurrence of Retinopathy of Prematurity After Intravitreal Ranibizumab Monotherapy: Timing and Risk Factors. Investig Ophthalmol Vis Sci. 2017;58:1719–25.
Zhang G, Yang M, Zeng J, Vakros G, Su K, Chen M, et al. Comparison Of Intravitreal Injection Of Ranibizumab Versus Laser Therapy For Zone II Treatment-Requiring Retinopathy Of Prematurity. Retina. 2017;37:710–7.
Yi Z, Su Y, Zhou Y, Zheng H, Ye M, Xu Y, et al. Effects of Intravitreal Ranibizumab in the Treatment of Retinopathy of Prematurity in Chinese Infants. Curr Eye Res. 2016;41:1092–7.
Feng J, Qian J, Jiang Y, Zhao M, Liang J, Yin H, et al. Efficacy of Primary Intravitreal Ranibizumab for Retinopathy of Prematurity in China. Ophthalmology. 2017;124:408–9.
Garcia Gonzalez JM, Snyder L, Blair M, Rohr A, Shapiro M, Greenwald M. Prophylactic peripheral laser and fluorescein angiography after bevacizumab for retinopathy of prematurity. Retina. 2018;38:764–72.
Shah PK, Subramanian P, Venkatapathy N, Chan R, Chiang MF, Campbell JP. Aggressive posterior retinopathy of prematurity in two cohorts of patients in South India: implications for primary, secondary, and tertiary prevention. JAAPOS. 2019;23:264.e1–264.
Yang XM, Zhao YX, Wang ZH, Liu L. Effect of anti-VEGF treatment on retinopathy of prematurity in Zone II Stage 3. Int J Ophthalmol. 2018;11:641–4.
Hu Q, Bai Y, Chen X, Huang L, Chen Y, Li X. Recurrence of Retinopathy of Prematurity in Zone II Stage 3+ after Ranibizumab Treatment: A Retrospective Study. J Ophthalmol. 2017;2017:5078565.
Martínez-Castellanos MA, González-H León A, Romo-Aguas JC, Gonzalez-Gonzalez LA. A proposal of an algorithm for the diagnosis and treatment of recurrence or treatment failure of retinopathy of prematurity after anti-VEGF therapy based on a large case series. Graefes Arch Clin Exp Ophthalmol. 2020;258:767–72.
Fidler M, Fleck BW, Stahl A, Marlow N, Chastain JE, Li J, et al. Ranibizumab population pharmacokinetics and free VEGF pharmacodynamics in preterm infants with retinopathy of prematurity in the RAINBOW trial. Transl Vis Sci Technol. 2020;9:43.
Acknowledgements
This work was supported by Science and Technology Development Fund (STDF), as capacity building grant for equipment. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
The study idea and design were conceived by RMB, WMG and MRB. Material preparation, data collection and analysis were performed by RMB, AEN, EAA and AGE. Preparation of the first draft of the manuscript was written by RMB and AGA. Final review of the manuscript was performed by WMG, AEN, AGA and MRB. All authors approved the final version of the manuscript that was submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bassiouny, R.M., Gaafar, W.M., El Nokrashy, A. et al. Clinical outcome following reinjection of Ranibizumab for reactivation of retinopathy of prematurity. Eye 36, 2137–2143 (2022). https://doi.org/10.1038/s41433-021-01814-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01814-5
This article is cited by
-
Characteristics of retinal vascularization in reactivated retinopathy of prematurity requiring treatment and clinical outcome after reinjection of ranibizumab
Scientific Reports (2024)
-
Ranibizumab
Reactions Weekly (2023)
-
Comparison of bevacizumab, ranibizumab and aflibercept in retinopathy of prematurity treatment
International Ophthalmology (2022)