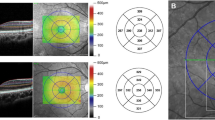
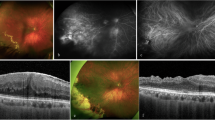
Abstract
Background/Aims
Optic pathway gliomas (OPGs) may cause progressive visual loss despite chemotherapy. Newer, less toxic treatments might be given earlier, depending on visual prognosis. We aimed to investigate the prognostic value of visual evoked potentials (VEP) and optical coherence tomography (OCT).
Methods
A retrospective study of OPG patients (treated 2003–2017) was conducted. Primary outcome was PEDIG category visual acuity in better and worse eyes (good < = 0.2, moderate 0.3–0.6 and poor > = 0.7 logMAR). Binary logistic regression analysis was used to identify predictors of these outcomes.
Results
60 patients (32 Neurofibromatosis type 1 [NF1] and 28 sporadic) had median presentation age 49 months (range 17–183) (NF1) and 27 months (range 4–92) (sporadic). Median follow up was 82 months (range 12–189 months). At follow up 24/32 (75%) of NF1 children and 14/28 (50%) of sporadic children had good better eye visual acuity and 11/32 (34%) of NF1 children and 15/28 (54%) of sporadics had poor worse eye acuity. Mean peripapillary retinal nerve fibre layer (RNFL) thickness predicted good better eye final acuity (OR 0.799, 95%CI 0.646–0.987, p = 0.038). Presenting with visual symptoms (OR 0.22 95% CI 0.001–0.508, p = 0.017) and poorer VEP scores (OR 2.35 95% CI 1.1–5.03, p = 0.027) predicted poor worse eye final acuity. 16 children had homonymous hemianopias at follow up, predicted by poor presenting binocular VEP score (OR 1.449 95%CI 1.052–1.995, p = 0.02).
Conclusions
We found that both RNFL thickness on OCT and VEP were useful in predicting future visual acuity and vision and potentially in planning treatment. We had a high prevalence of homonymous hemianopia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Central Brain Tumor Registry of the United States. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2010.
Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997;41:143–9.
Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, et al. Alex’s Lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncol. 2015;16:x1–36.
Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–98.
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-Oncol. 2014;16:iv1–63.
Blazo MA, Lewis RA, Chintagumpala MM, Frazier M, McCluggage C, Plon SE. Outcomes of systematic screening for optic pathway tumors in children with Neurofibromatosis Type 1. Am J Med Genet A. 2004;127A:224–9.
Chen Y-H, Gutmann DH. The molecular and cell biology of pediatric low-grade gliomas. Oncogene. 2014;33:2019–26.
Fried I, Tabori U, Tihan T, Reginald A, Bouffet E. Optic pathway gliomas: a review. CNS Oncol. 2013;2:143–59.
Czyzyk E, Józwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18:471–8.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89:1–6.
Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro-Oncol. 2012;14:790–7.
Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28:262–70.
Lewis RA, Gerson LP, Axelson KA, Riccardi VM, Whitford RP. von Recklinghausen neurofibromatosis. II. Incid Opt gliomata Ophthalmol. 1984;91:929–35.
Liu GT, Brodsky MC, Phillips PC, Belasco J, Janss A, Golden JC, et al. Optic radiation involvement in optic pathway gliomas in neurofibromatosis. Am J Ophthalmol. 2004;137:407–14.
Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–54.
Campagna M, Opocher E, Viscardi E, Calderone M, Severino SM, Cermakova I, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55:1083–8.
Rakotonjanahary J, Gravier N, Lambron, De Carli E, Toulgoat F, Delion M, et al. Long-term visual acuity in patients with optic pathway glioma treated during childhood with up-front BB-SFOP chemotherapy—Analysis of a French pediatric historical cohort. PLoS One 2019. 2019;14:e0212107. Epub 2019 Mar 8
Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol. 2011;31:269–78.
Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol. 2014;116:341–7. Jan
Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132:111–4. https://doi.org/10.1001/jamaophthalmol.2013.5819.
Lu VM, Welby JP, Nesvick CL, Daniels DJ. Efficacy and safety of bevacizumab in progressive pediatric low-grade glioma: a systematic review and meta-analysis of outcome rates. Neurooncol Pract. 2020;7:359–68. https://doi.org/10.1093/nop/npz076. Epub 2020 Feb 3.
Zhukova N, Rajagopal R, Lam A, Coleman L, Shipman P, Walwyn T, et al. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019;8:40–50. https://doi.org/10.1002/cam4.1799. Epub 2018 Dec 19.
Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 2019;20:1011–22. https://doi.org/10.1016/S1470-2045(19)30277-3. Epub 2019 May 28.
Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin Cancer Res. 2019;25:7303–11. https://doi.org/10.1158/1078-0432.CCR-19-2177.
Avery RA, Liu GT, Fisher MJ, Quinn GE, Belasco JB, Phillips PC, et al. Retinal nerve fibre layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151:542–9.
Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014;55:1402–8. https://doi.org/10.1167/iovs.13-13119.
Chang BC, Mirabella G, Yagev R, Banh M, Mezer E, Parkin PC, et al. Screening and diagnosis of optic pathway gliomas in children with neurofibromatosis type 1 by using sweep visual evoked potentials. Invest Ophthalmol Vis Sci. 2007;48:2895–902.
Parrozzani R, Clementi M, Kotsafti O, Miglionico G, Trevisson E, Orlando G, et al. Optical coherence tomography in the diagnosis of optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54:8112–8.
Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46:2253–9.
Azizi AA, Walker DA, Liu JF, Sehested A, Jaspan T, Pemp B, et al. NF1 optic pathway glioma. Analysing risk factors for visual outcome and indications to treat.; SIOPE NF1 OPG Nottingham, UK, Workshop 2014. Neuro Oncol. 2020;6:noaa153.
Falzon K, Drimtzias E, Picton S, Simmons I. Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) Low-Grade Glioma 2004 trial UK cohort. Br J Ophthalmol. 2018;102:1367–71. https://doi.org/10.1136/bjophthalmol-2017-311305. Epub 2018 Jan 17
Dodge HW, Love JG, Craig WM, Dockerty MB, Kearns TP, Holman CB, et al. Gliomas of the optic nerves. AMA Arch Neurol Psychiatry. 1958;79:607–21.
Yanni SE, Wang J, Cheng CS, Locke KI, Wen Y, Birch DG, et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol. 2013;155:354–60.e1. https://doi.org/10.1016/j.ajo.2012.08.010. Epub 2012 Nov 3
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A, et al. International Society for Clinical Electrophysiology of V. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol. 2016;133:1–9.
Ophthalmic statistics note 1: unit of analysis, Bunce C, Patel KV, Xing W, Freemantle N, Doré CJ. Ophthalmic statistics group. Br J Ophthalmol 2014;98:408–12. https://doi.org/10.1136/bjophthalmol-2013-304587. Epub 2013 Dec 19.
Vision impairment and blindness [Internet]. Who.int. 2019 [cited 12 August 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment
Hamilton R, Bach M, Heinrich SP, Hoffmann MB, Odom JV, McCulloch DL, et al. VEP estimation of visual acuity: a systematic review. Doc Ophthalmol. 2020. https://doi.org/10.1007/s10633-020-09770-3. Online ahead of print.
Funding
VS was funded by a grant from Great Ormond Street Hospital Children’s Charity.
Author information
Authors and Affiliations
Contributions
RB, DAT and DH conceived and designed study, RB analysed the data and wrote the paper with input from DAT and DH, all the other authors contributed to data collection and entry and reviewed the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bowman, R., Walters, B., Smith, V. et al. Visual outcomes and predictors in optic pathway glioma: a single centre study. Eye 37, 1178–1183 (2023). https://doi.org/10.1038/s41433-022-02096-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-022-02096-1
This article is cited by
-
Complications and visual outcomes following surgical resection of pediatric optic pathway/hypothalamic gliomas: a systematic review and meta-analysis
Child's Nervous System (2024)
-
The role of visual electrodiagnostics in management of children with neurofibromatosis type 1
Documenta Ophthalmologica (2023)