Abstract
Background/objectives
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder. Despite promising advances with anti-VEGF therapy, select patients are unresponsive to therapy. A precision medicine-based approach for therapeutic decision-making based on underlying biomarkers may facilitate treatment based on the underlying pathway. This study aims to identify the baseline and longitudinal cytokine profiles of RVO-related macular oedema and correlating these expression profiles with higher order OCT features using a novel retinal segmentation and feature extraction platform.
Subjects/methods
The IMAGINE study is a post-hoc assessment of aqueous humour cytokines with correlation to higher level analysis of imaging studies. OCT scans underwent machine learning enhanced segmentation of the internal limiting membrane (ILM), ellipsoid zone (EZ) and retinal pigment epithelium (RPE), as well as evaluating volumetric fluid metrics. Samples of aqueous humour were obtained at baseline, as well as months 4 and 9 prior to treatment. These samples were analysed for the expression of multiple cytokines. Patients were divided into Responders and Non-Responders based on OCT profiles. Additionally, patients were categorised as a Rebounder if their CST increased by 50% after initial improvement.
Results
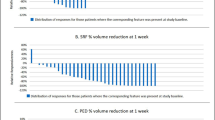
Twenty-six eyes were included. The OCT-based response schema identified 21 Responders (81%) and 5 Non-Responders (19%). VEGF levels directly correlated with intraretinal fluid volume and angiogenin was inversely correlated with fluid indices. Multiple cytokines, including ANGPTL4, were directly correlated with ellipsoid zone disruption. The baseline VEGF levels were significantly higher in all responders compared to Non-Responders (p = 0.02). Rebounders tended to have significantly decreased levels of angiogenin and TIMP-1 (p = 0.019, p = 0.015).
Conclusions
Cytokine expression was linked to specific OCT features and treatment response in RVO. Identification of an imaging phenotype that could serve as a surrogate for underlying active disease pathways could enhance treatment decision-making and precision medicine.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Additional data are available upon request.
References
Michels RG, Gass JD. The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:Op166–77.
Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56:281–99.
Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 1996;114:1243–7.
Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. discussion 41-3
Cheung N, Klein R, Wang JJ, Cotch MF, Islam AF, Klein BE, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Investig Ophthalmol Vis Sci. 2008;49:4297–302.
Noma H, Mimura T, Yasuda K, Shimura M. Functional-morphological parameters, aqueous flare and cytokines in macular oedema with branch retinal vein occlusion after ranibizumab. Br J Ophthalmol. 2017;101:180–5.
Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140:256–61.
Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of macular edema. Ophthalmologica J Int d’ophtalmologie. 2010;224:8–15.
Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–12.e1.
Varma R, Bressler NM, Suner I, Lee P, Dolan CM, Ward J, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology. 2012;119:2108–18.
Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802–9.
Modi A, Sharma K, Sudhakar NP, Yadav NK. Aqueous humor cytokines and therapeutic customization in nonresponding macular edema secondary to retinal vein occlusion. Retin Cases Brief Rep. 2021;15:127–30.
Zeng Y, Cao D, Yu H, Zhuang X, Yang D, Hu Y, et al. Comprehensive analysis of vitreous chemokines involved in ischemic retinal vein occlusion. Mol Vis. 2019;25:756–65.
Narayanan R, Stewart MW, Chhablani J, Panchal B, Pappuru RR, Das T, et al. Baseline morphological characteristics as predictors of final visual acuity in patients with branch retinal vein occlusions: MARVEL report no. 3. Indian J Ophthalmol. 2018;66:1291–4.
Chan EW, Eldeeb M, Sun V, Thomas D, Omar A, Kapusta MA, et al. Disorganization of retinal inner layers and ellipsoid zone disruption predict visual outcomes in central retinal vein occlusion. Ophthalmol Retin. 2019;3:83–92.
Kotake O, Noma H, Yasuda K, Motohashi R, Goto H, Shimura M. Comparing cytokine kinetics between ranibizumab and aflibercept in central retinal vein occlusion with macular edema. Ophthalmic Res. 2018:1–8.
Wykoff CC, Ou WC, Wang R, Brown DM, Cone C, Zamora D, et al. Peripheral laser for recalcitrant macular edema owing to retinal vein occlusion: the WAVE trial. Ophthalmology. 2017;124:919–21.
Ehlers JP, Uchida A, Hu M, Figueiredo N, Kaiser PK, Heier JS, et al. Higher-order assessment of OCT in diabetic macular edema from the VISTA study: ellipsoid zone dynamics and the retinal fluid index. Opthalmol Retina. 2019;3:1056–66.
Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualisation of outer retinal integrity with optical coherence tomography. Br J Ophthalmol. 2016;100:295–9.
Ehlers JP, Clark J, Uchida A, Figueiredo N, Babiuch A, Talcott KE, et al. Longitudinal higher-order OCT assessment of quantitative fluid dynamics and the total retinal fluid index in neovascular AMD. Transl Vis Sci Technol 2021;10:29.
Ehlers JP, Zahid R, Kaiser PK, Heier JS, Brown DM, Meng X, et al. Longitudinal assessment of ellipsoid zone integrity, subretinal hyperreflective material, and subretinal pigment epithelium disease in neovascular age-related macular degeneration. Ophthalmol Retina. 2021;5:1204–13.
Abraham JR, Wykoff CC, Arepalli S, Lunasco L, Yu HJ, Hu M, et al. Aqueous cytokine expression and higher-order OCT biomarkers: assessment of the anatomic-biologic bridge in the IMAGINE DME study. Am J Ophthalmol. 2021;222:328–39.
Ehlers JP, Uchida A, Hu M, Figueiredo N, Kaiser PK, Heier JS, et al. Higher-order assessment of OCT in diabetic macular edema from the VISTA study: ellipsoid zone dynamics and the retinal fluid index. Ophthalmol Retin. 2019;3:1056–66.
Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115–28.
Campochiaro PA, Hafiz G, Mir TA, Scott AW, Zimmer-Galler I, Shah SM, et al. Pro-permeability factors in diabetic macular edema; the diabetic macular edema treated with ozurdex trial. Am J Ophthalmol. 2016;168:13–23.
Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye. 2008;22:42–8.
Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011;55:256–63.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7.
Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol Adv Ophthalmol. 1999;97:217–28.
Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783–9.
Noma H, Mimura T, Eguchi S. Association of inflammatory factors with macular edema in branch retinal vein occlusion. JAMA Ophthalmol. 2013;131:160–5.
Funatsu H, Noma H, Mimura T, Eguchi S. Vitreous inflammatory factors and macular oedema. Br J Ophthalmol. 2012;96:302–4.
Lim JW. Intravitreal bevacizumab and cytokine levels in major and macular branch retinal vein occlusion. Ophthalmol J Int d’ophtalmologie Int J Ophthalmol Z fur Augenheilkd. 2011;225:150–4.
Matsushima R, Noma H, Yasuda K, Goto H, Shimura M. Role of cytokines in ranibizumab therapy for macular edema in patients with central retinal vein occlusion. J Ocul Pharmacol Ther. 2019;35:407–12.
Wen J, Jiang Y, Zheng X, Zhou Y. Six-month changes in cytokine levels after intravitreal bevacizumab injection for diabetic macular oedema and macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2015;99:1334–40.
Park SP, Ahn JK. Changes of aqueous vascular endothelial growth factor and pigment epithelium-derived factor following intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Clin Exp Ophthalmol. 2009;37:490–5.
Funk M, Kriechbaum K, Prager F, Benesch T, Georgopoulos M, Zlabinger GJ, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Investig Ophthalmol Vis Sci. 2009;50:1025–32.
Noma H, Mimura T, Yasuda K, Shimura M. Role of soluble vascular endothelial growth factor receptor signaling and other factors or cytokines in central retinal vein occlusion with macular edema. Investig Ophthalmol Vis Sci. 2015;56:1122–8.
Marek N, Raczyńska K, Siebert J, Myśliwiec M, Zorena K, Myśliwska J, et al. Decreased angiogenin concentration in vitreous and serum in proliferative diabetic retinopathy. Microvasc Res. 2011;82:1–5.
Coroniti R, Fario R, Nuno DJ, Otvos L, Scolaro L, Surmacz E. Designer leptin receptor antagonist allo-aca inhibits VEGF effects in ophthalmic neoangiogenesis models. Front Mol Biosci. 2016;3:67.
Noma H, Funatsu H, Mimura T, Harino S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87–93.
Sugimoto M, Cutler A, Shen B, Moss SE, Iyengar SK, Klein R, et al. Inhibition of EGF signaling protects the diabetic retina from insulin-induced vascular leakage. Am J Pathol. 2013;183:987–95.
Kim HS, Vargas A, Eom YS, Li J, Yamamoto KL, Craft CM, et al. Tissue inhibitor of metalloproteinases 1 enhances rod survival in the rd1 mouse retina. PloS One. 2018;13:e0197322.
Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Investig. 1998;101:1478–87.
Funding
Funding was in part provided by NIH/NEI K23-EY022947-01A1 (JPE).
Author information
Authors and Affiliations
Contributions
Study design: JPE, CCW; Funding: CCW, JPE; Data Acquisition: JRA, SAA, LL, HJY, CCW, JPE; Manuscript drafting: SA; Manuscript Editing/Revision: All authors; Supervision: JPE, CCW, SKS, JLR; Statistical support: MH.
Corresponding author
Ethics declarations
Competing interests
CCW has research support from the following: Adverum, Allergan, Apellis, Clearside, EyePoint, Genentech/Roch, Neurotech, Novartis, Opthea, Regeneron, Regenxbio, Samsung, Santen; is a consultant for the following: Alimera Sciences, Allegro, Allergan, Alynylam, Apellis, Bayer, Clearside, D.O.R.C., EyePoint, Genentech/Roche, Kodiak, Notal Vision, Novartis, ONL Therapeutics, PolyPhotonix, RecensMedical, Regeneron, Regenxbio, Santen; and is a speaker for Regeneron. SKS has research support from Regeneron, Allergan, and Gilead; is a consultant for Bausch and Lomb, Novartis, and Regeneron. DMB has research support from the following: Adverum, Allergan, Apellis, Clearside, Genentech/Roch, Novartis, Opthea, Regeneron, Regenxbio, Samsung, Santen; is a consultant for the following: Regeneron, Bayer, Senju, Allergan, Optos, Zeiss, Heidelberg, OHR, Biotime, Gemini, Genentech/Roche, Novartis, Apellis, Regenxbio, Chengdu Kanghong Biotechnology. JPE has research support from the following: Aerpio, Alcon, Thrombogenics/Oxurion, Regeneron, Genentech, Novartis, Allergan; is a consultant for the following: Aerpio, Alcon, Allegro, Allergan, Adverum, Genentech/Roche, Novartis, Thrombogenics/Oxurion, Leica, Zeiss, Regeneron, Stealth; and holds a patent with Leica. JRA, SA, LL, HJY, MH, JLR have no financial disclosures to report. The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arepalli, S., Wykoff, C.C., Abraham, J.R. et al. Longitudinal analysis of aqueous humour cytokine expression and OCT-based imaging biomarkers in retinal vein occlusions treated with anti-vascular endothelial growth factor therapy in the IMAGINE study. Eye 37, 1928–1935 (2023). https://doi.org/10.1038/s41433-022-02265-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-022-02265-2
This article is cited by
-
Potential sustained benefits of early targeted panretinal photocoagulation in combination with anti-VEGF in macular edema secondary to retinal vein occlusion: 48-month results of a retrospective comparative study
BMC Ophthalmology (2025)
-
Aqueous humor cytokine levels in retinal vein occlusion patients with suboptimal response to anti-VEGF therapy and the correlation with OCT imaging biomarkers
European Journal of Medical Research (2025)