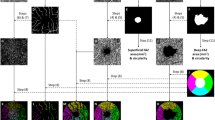
Abstract
Background/objectives
Study of retinal structure based on optical coherence tomography (OCT) data can facilitate early diagnosis of relapsing-remitting multiple sclerosis (RRMS). Although artificial intelligence can provide highly reliable diagnoses, the results obtained must be explainable.
Subjects/methods
The study included 79 recently diagnosed RRMS patients and 69 age matched healthy control subjects. Thickness (Avg) and inter-eye difference (Diff) features are obtained in 4 retinal layers using the posterior pole protocol. Each layer is divided into six analysis zones. The Support Vector Machine plus Recursive Feature Elimination with Leave-One-Out Cross Validation (SVM-RFE-LOOCV) approach is used to find the subset of features that reduces dimensionality and optimises the performance of the classifier.
Results
SVM-RFE-LOOCV was used to identify OCT features with greatest capacity for early diagnosis, determining the area of the papillomacular bundle to be the most influential. A correlation was observed between loss of layer thickness and increase in functional disability. There was also greater functional deterioration in patients with greater asymmetry between left and right eyes. The classifier based on the top-ranked features obtained sensitivity = 0.86 and specificity = 0.90.
Conclusions
There was consistency between the features identified as relevant by the SVM-RFE-LOOCV approach and the retinotopic distribution of the retinal nerve fibres and the optic nerve head. This simple method contributes to implementation of an assisted diagnosis system and its accuracy exceeds that achieved with magnetic resonance imaging of the central nervous system, the current gold standard. This paper provides novel insights into RRMS affectation of the neuroretina.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The data collected and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol [Internet]. 2018;17:162–73. https://linkinghub.elsevier.com/retrieve/pii/S1474442217304702.
van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, Runia TF, Jafari N, Samijn JP, et al. Application of the 2017 revised Mcdonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol. 2018;75:1392.
Gaitán MI, Sanchez M, Farez MF, Fiol MP, Ysrraelit MC, Solomon AJ, et al. The frequency and characteristics of multiple sclerosis misdiagnosis in Latin America: A referral center study in Buenos Aires, Argentina. Mult Scler J. 2022;28:1373–81.
Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol [Internet]. 2017;16:797–812. https://linkinghub.elsevier.com/retrieve/pii/S1474442217302788.
Ortiz M, Mallen V, Boquete L, Sánchez-Morla EM, Cordón B, Vilades E, et al. Diagnosis of multiple sclerosis using optical coherence tomography supported by artificial intelligence. Mult Scler Relat Disord. 2023;74:104725.
Paul F, Calabresi PA, Barkhof F, Green AJ, Kardon R, Sastre‐Garriga J, et al. Optical coherence tomography in multiple sclerosis: a 3‐year prospective multicenter study. Ann Clin Transl Neurol [Internet]. 2021;8:2235–51. https://onlinelibrary.wiley.com/doi/10.1002/acn3.51473.
Petzold A, Chua SYL, Khawaja AP, Keane PA, Khaw PT, Reisman C, et al. Retinal asymmetry in multiple sclerosis. Brain [Internet]. 2021;144:224–35. https://academic.oup.com/brain/article/144/1/224/60127953.
Nij Bijvank J, Uitdehaag BMJ, Petzold A. Retinal inter-eye difference and atrophy progression in multiple sclerosis diagnostics. J Neurol Neurosurg Psychiatry [Internet]. 2022;93:216–9. https://jnnp.bmj.com/lookup/doi/10.1136/jnnp-2021-327468.
Patil SA, Joseph B, Tagliani P, Sastre-Garriga J, Montalban X, Vidal-Jordana A, et al. Longitudinal stability of inter-eye differences in optical coherence tomography measures for identifying unilateral optic nerve lesions in multiple sclerosis. J Neurol Sci. 2023;449:120669.
Loh HW, Ooi CP, Seoni S, Barua PD, Molinari F, Acharya UR. Application of explainable artificial intelligence for healthcare: a systematic review of the last decade (2011–2022). Comput Methods Prog Biomed. 2022;226:107161.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389–422.
Chylack LT. The lens opacities classification system III. Arch Ophthalmol. 1993;111:831.
Petzold A, Albrecht P, Balcer L, Bekkers E, Brandt AU, Calabresi PA, et al. Artificial intelligence extension of the OSCAR‐IB criteria. Ann Clin Transl Neurol. 2021;8:1528–42.
Vabalas A, Gowen E, Poliakoff E, Casson AJ. Machine learning algorithm validation with a limited sample size. PLoS One. 2019;14:e0224365.
Al-Nosairy K, Horbrügger M, Schippling S, Wagner M, Haghikia A, Pawlitzki M, et al. Structure–function relationship of retinal ganglion cells in multiple sclerosis. Int J Mol Sci. 2021;22:3419.
Satue M, Obis J, Rodrigo MJ, Otin S, Fuertes MI, Vilades E. et al. Optical coherence tomography as a biomarker for diagnosis, progression, and prognosis of neurodegenerative diseases.J Ophthalmol [Internet].2016;2016:8503859 http://www.ncbi.nlm.nih.gov/pubmed/27840739.
Ciftci Kavaklioglu B, Erdman L, Goldenberg A, Kavaklioglu C, Alexander C, Oppermann HM, et al. Machine learning classification of multiple sclerosis in children using optical coherence tomography. Mult Scler J. 2022;28:2253–62.
Hernandez M, Ramon-Julvez U, Vilades E, Cordon B, Mayordomo E, Garcia-Martin E. Explainable artificial intelligence toward usable and trustworthy computer-aided early diagnosis of multiple sclerosis from optical coherence tomography. PLoS One. 2023;18:e0289495.
Garcia-Martin E, Rodriguez-Mena D, Herrero R, Almarcegui C, Dolz I, Martin J, et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurol [Internet]. 2013;81:76–83. http://www.neurology.org/cgi/doi/10.1212/WNL.0b013e318299ccd9.
Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol [Internet]. 2016;15:574–84. https://linkinghub.elsevier.com/retrieve/pii/S1474442216000685.
Giedraitiene N, Drukteiniene E, Kizlaitiene R, Cimbalas A, Asoklis R, Kaubrys G. Cognitive decline in multiple sclerosis is related to the progression of retinal atrophy and presence of oligoclonal bands: a 5-Year follow-up study. Front Neurol. 2021;12:678735.
Frau J, Fenu G, Signori A, Coghe G, Lorefice L, Barracciu MA, et al. A cross-sectional and longitudinal study evaluating brain volumes, RNFL, and cognitive functions in MS patients and healthy controls. BMC Neurol. 2018;18:67.
Pérez Del Palomar A, Cegoñino J, Montolío A, Orduna E, Vilades E, Sebastián B, et al. Swept source optical coherence tomography to early detect multiple sclerosis disease. The use of machine learning techniques. PLoS One [Internet]. 2019;14:e0216410. http://www.ncbi.nlm.nih.gov/pubmed/31059539.
Montolío A, Cegoñino J, Garcia-Martin E, Pérez del Palomar A. Comparison of machine learning methods using spectralis OCT for diagnosis and disability progression prognosis in multiple sclerosis. Ann Biomed Eng. 2022;50:507–28.
Manogaran P, Hanson J, Olbert E, Egger C, Wicki C, Gerth-Kahlert C, et al. Optical coherence tomography and magnetic resonance imaging in multiple sclerosis and neuromyelitis optica spectrum disorder. Int J Mol Sci. 2016;17:1894.
Wildner P, Stasiołek M, Matysiak M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult Scler Relat Disord. 2020;37:101452.
Acknowledgements
Thanks to Dr. Luis E Pablo for his help with the use of the devices.
Funding
This study was supported by Carlos III Health Institute grants PI17/01726, PI18/1275 and PI20/00437, by the Inflammatory Disease Network (RICORS) (RD21/0002/0050) (Carlos III Health Institute and co-funded by the European Union “NextGenerationEU/PRTR), and by project reference UAH-GP2022-2 funded by the University of Alcalá Proprietary Research Programme. The funding organisations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
FJD-M has contributed to methodology, investigation, formal analysis and writing original draft. MO has contributed to methodology, investigation, software, formal analysis, data curation and review and editing. AP has contributed to methodology and investigation. LB has contributed to conceptualisation, formal analysis, data curation, writing original draft and review and editing. EMS-M has contributed to formal analysis, data curation, and review and editing. DJ-H has contributed to methodology, investigation, software, data curation and review and editing. JMM has contributed to methodology, investigation, software, data curation and review and editing. RB has contributed to methodology, investigation, software, data curation and review and editing. EV has contributed to methodology, investigation, software, data curation and review and editing. EG-M has contributed to conceptualisation, formal analysis, data curation, writing original draft and review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dongil-Moreno, F.J., Ortiz, M., Pueyo, A. et al. Diagnosis of multiple sclerosis using optical coherence tomography supported by explainable artificial intelligence. Eye 38, 1502–1508 (2024). https://doi.org/10.1038/s41433-024-02933-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-024-02933-5