Abstract
Purpose
Bisphosphonates (BPs) are first line agents commonly used in the management of osteoporosis. There have been two case reports that have suggested a possible link between BPs and acute angle closure (AAC). In the absence of any large epidemiologic studies, we sought to determine the risk of AAC and OAG with bisphosphonate use in patients with osteoporosis.
Methods
This was a retrospective cohort study with a case control analysis from 2008–2018. The study used the PharMetrics Plus Database (IQVIA, USA) which captures health claims for over 150 million unique patients, with fully adjudicated pharmacy and medical claims, and represents all geographic areas of the United States. 208,111 patients with osteoporosis were included in the study. AAC and OAG cases were defined by an ICD-9/10 code and had to have at least one prescription for bisphosphonate every 3 months in the year prior to the index date. The date of the first event of AAC was designated as the index date.
Results
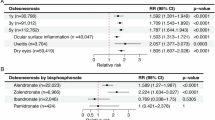
Bisphosphonate users were more likely to develop AAC than non-users (adjusted IRR = 1.78, 95%CI [1.05–3.01]). In particular, those on risedronate were more likely to develop AAC compared to patients who used other formulations of bisphosphonates (adjusted IRRs = 2.12, 95%CI [1.05–3.01]). There was no risk for OAG with bisphosphonate use.
Conclusions
Patients with osteoporosis who used BPs were at a higher risk for AAC compared to those who did not, and those who were on risedronate were more likely to develop AAC compared to patients who used other types of bisphosphonates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11.
Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep. 2021;11:13762.
Kuang TM, Xirasagar S, Kao YW, Shia BC, Lin HC. Association of systemic hypertension with primary open-angle glaucoma: a population-based case-control study. Am J Ophthalmol. 2020;218:99–104.
Ah-Kee EY, Egong E, Shafi A, Lim LT, Yim JL. A review of drug-induced acute angle closure glaucoma for non-ophthalmologists. Qatar Med J. 2015;2015:6.
Tham YC, Cheng CY. Associations between chronic systemic diseases and primary open angle glaucoma: an epidemiological perspective. Clin Exp Ophthalmol. 2017;45:24–32.
Fogel HA, Jenis LG. The economic burden of osteoporosis. In: Razi AE, Hershman SH, editors. Vertebral compression fractures in osteoporotic and pathologic bone: a clinical guide to diagnosis and management. Cham: Springer International Publishing; 2020. p. 21–9. Available from: https://doi.org/10.1007/978-3-030-33861-9_3.
Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. Available from: https://pubmed.ncbi.nlm.nih.gov/28293453.
McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, et al. Efficacy and safety of risedronate 150-mg once a month in the treatment of postmenopausal osteoporosis: 2-year data. Osteoporos Int. 2013;24:293–9.
Schnitzer TJ. Update on alendronate for osteoporosis: once-weekly dosing. Expert Opin Pharmacother. 2001;2:1461–72.
Maricic M. The role of zoledronic acid in the management of osteoporosis. Clin Rheumatol. 2010;29:1079–84.
Khan A, Lascaratos G, Rane-Malcolm T, Sanders R. A rare case of zolendronate infusion complication leading to glaucoma filtration surgery. Clin Ophthalmol. 2011;5:1147–9. Available from: https://pubmed.ncbi.nlm.nih.gov/21887097.
Thng ZX, Li ZK, Gan NY. Bisphosphonate-induced bilateral anterior uveitis and choroidal effusions with secondary angle closure. Retin Cases Brief Rep. 2021;15:552–5.
IQVIA. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.greygreenmedia.com/app/uploads/sites/2/2020/09/IQVIA-PharMetrics-Plus-US-Fact-Sheet.pdf. 2020. IQVIA PharMectrics Plus Fact sheet.
Aurich-Barrera B, Wilton L, Harris S, Shakir SAWhttps://pubmed.ncbi.nlm.nih.gov/16454542/. Ophthalmological events in patients receiving risedronate: summary of information gained through follow-up in a prescription-event monitoring study in England. Drug Saf. 2006;29:151–60. [cited 2022 Oct 9] Available from: .
Mbekeani JN, Slamovits TL, Schwartz BH, Sauer HL. Ocular inflammation associated with alendronate therapy. Arch Ophthalmol [Internet]. 1999;117:837–8. [cited 2022 Oct 9] Available from: https://pubmed.ncbi.nlm.nih.gov/10369603/.
Etminan M, Forooghian F, Maberley D. Inflammatory ocular adverse events with the use of oral bisphosphonates: a retrospective cohort study. CMAJ Can Med Assoc J. 2012;184:E431–4. May 15
Fraunfelder FW. Ocular side effects associated with bisphosphonates. Drugs Today. 2003;39:829–35. [cited 2022 Oct 9] Available from: https://pubmed.ncbi.nlm.nih.gov/14702129/.
Pimentel MA, Browne EN, Janardhana PM, Borkar DS, Tham VM, Uchida A, et al. Assessment of the accuracy of using ICD-9 codes to identify uveitis, herpes zoster ophthalmicus, scleritis, and episcleritis. JAMA Ophthalmol. 2016;134:1001–6.
Palestine AG, Merrill PT, Saleem SM, Jabs DA, Thorne JE. Assessing the precision of ICD-10 codes for uveitis in 2 electronic health record systems. JAMA Ophthalmol. 2018;136:1186–90.
Nakayama LF, Ribeiro LZ, Dychiao RG, Zamora YF, Regatieri CVS, Celi LA, et al. Artificial intelligence in uveitis: a comprehensive review. Surv Ophthalmol. 2023;68:669–77.
Paikal D, Yu F, Holland GN, Coleman AL. Coding of glaucoma for patients with uveitis in the medicare database. J Glaucoma. 2006;15:13–6.
Chiu HC, Chiu CY, Yang RS, Chan DC, Liu SH, Chiang CK. Preventing muscle wasting by osteoporosis drug alendronate in vitro and in myopathy models via sirtuin-3 down-regulation. J Cachexia Sarcopenia Muscle. 2018;9:585–602.
Huang CF, Shiao MS, Mao TY. Retrospective study of the effects of zoledronic acid on muscle mass in osteoporosis patients. Drug Des Dev Ther. 2021;15:3711–5.
Billington EO, Reid IR. Benefits of bisphosphonate therapy: beyond the skeleton. Curr Osteoporos Rep. 2020;18:587–96.
Wu L, Zhu L, Shi WH, Zhang J, Ma D, Yu B. Zoledronate inhibits the proliferation, adhesion and migration of vascular smooth muscle cells. Eur J Pharm. 2009;602:124–31.
Arun MZ, Reel B, Sala-Newby GB, Bond M, Tsaousi A, Maskell P, et al. Zoledronate upregulates MMP-9 and -13 in rat vascular smooth muscle cells by inducing oxidative stress. Drug Des Dev Ther. 2016;10:1453–60.
Funding
This study was funded by the Glaucoma Research Society of Canada Project Grant.
Author information
Authors and Affiliations
Contributions
Conceptualization: BH, RD, ME, BE. Data curation: BH, ME. Formal analysis: ME. Funding acquisition: BH, RD, ME, BE. Investigation: BH, ME, BE. Methodology: BH, RD, ME, BE. Project administration: BH, ME, BE. Resources: BH, RD, ME, BE. Software: ME. Supervision: ME, BE. Validation: BH, ME, BE. Visualization: BH, ME, BE. Roles/Writing—original draft: BH. Writing—review & editing: BH, ME, BE.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, B., Etminan, M., Darwich, R. et al. Risk of glaucoma with bisphosphonate use in patients with osteoporosis: a case-control study. Eye 39, 1160–1164 (2025). https://doi.org/10.1038/s41433-024-03574-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-024-03574-4
This article is cited by
-
Bisphosphonates increase risk of acute angle closure glaucoma
Reactions Weekly (2025)