Abstract
Purpose
To investigate primary suspect drugs and identify a potential association between medication use and Tubulointerstitial Nephritis and Uveitis (TINU).
Methods
A retrospective pharmacovigilance study was conducted using the Food and Drug Administration (FDA) Adverse Events Database (FAERS) from Q1 2004 to Q2 2024, focusing on patient demographics and statistical signal detection. A qualitative analysis assessed patient demographics. To ascertain if these reports yielded statistically significant signals, we used the proportional reporting ratio (PRR), chi-squared with Yates’ correction (χ2), reporting odds ratio (ROR), empirical Bayes geometric mean (EBGM), and information component (IC).
Results
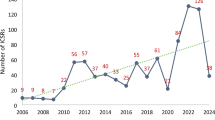
One hundred twenty-six adverse reports for TINU were identified, along with 37 primary suspect drugs from the FAERS database. The mean age of patients was 30.05 ± 20.88 years. Most reports were of female patients (n = 67, 53%). Of the 37 primary suspect drugs, lamotrigine (n = 35, 27%) and diclofenac (n = 15, 12%) were the most frequently reported suspect drugs. The signal detection analysis also identified positive signals and potential causality for both drugs. Lamotrigine demonstrated the strongest positive signal (PRR = 90.09, χ2 = 3001.58, ROR 95% CI: 124.35 [84.20-183.66], EBGM [EBGM05]: 70.98 [50.21], IC [IC05]: 5.32 [4.83]), followed by diclofenac (PRR = 24.21, χ2 = 312.49, ROR 95% CI: 27.35 [15.95-46.90], EBGM [EBGM05]: 4.11 [2.47], IC [IC05]: 3.79 [2.99]).
Conclusion
Our study identified 37 primary suspect drugs associated with TINU, with lamotrigine and diclofenac showing the strongest statistical signals. Lamotrigine demonstrated the highest association, suggesting a potential drug-related risk for developing TINU, particularly in younger patients and females. Further research is warranted to explore causality and underlying mechanisms in these associations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated for the statistical analysis and signal detection analysis are available from the corresponding author upon reasonable request.
References
Amaro D, Carreño E, Steeples LR, Oliveira-Ramos F, Marques-Neves C, Leal I. Tubulointerstitial nephritis and uveitis (TINU) syndrome: a review. Br J Ophthalmol. 2020;104:742–7. https://doi.org/10.1136/bjophthalmol-2019-314926.
Mandel M, Elhusseiny AM, Davidson SL, Rockter A, Levin AV, Huang LC, et al. Clinical outcomes in paediatric tubulointerstitial nephritis and uveitis syndrome (TINU). Eye. 2024; https://doi.org/10.1038/s41433-024-03286-9.
Mackensen F, Heiko B. Tubulointerstitial nephritis and uveitis syndrome. Curr Opin Ophthalmol. 2009;20:525–31. https://doi.org/10.1097/ICU.0b013e3283318f9a.
Provencher LM, Fairbanks AM, Abramoff MD, Syed NA. Urinary β2-microglobulin and disease activity in patients with tubulointerstitial nephritis and uveitis syndrome. J Ophthalmic Inflamm Infect. 2018;8:24. https://doi.org/10.1186/s12348-018-0166-3.
Mandeville JTH, Levinson RD, Holland GN. The Tubulointerstitial Nephritis and Uveitis Syndrome. Surv Ophthalmol. 2001;46:195–208. https://doi.org/10.1016/S0039-6257(01)00261-2.
Okafor LO, Hewins P, Murray PI, Denniston AK. Tubulointerstitial nephritis and uveitis (TINU) syndrome: a systematic review of its epidemiology, demographics and risk factors. Orphanet J Rare Dis. 2017;12:128. https://doi.org/10.1186/s13023-017-0677-2.
Ha A, Langroudi AP, Eisenberg ML. What is the validity of the Federal Adverse Event Reporting System in contemporary clinical research?. J Sex Med. 2024;21:744–5. https://doi.org/10.1093/jsxmed/qdae072.
Fusaroli M, Giunchi V, Battini V, Puligheddu S, Khouri C, Carnovale C, et al. Enhancing Transparency in Defining Studied Drugs: The Open-Source Living DiAna Dictionary for Standardizing Drug Names in the FAERS. Drug Saf. 2024;47:271–84. https://doi.org/10.1007/s40264-023-01391-4.
Groat B, Karpow C Datamining Medication Error Reports in the FDA Adverse Event Reporting System (FAERS) to Supplement Strategies for Identifying Potential Safety Signals. 2021.
Practical Aspects of Signal Detection in Pharmacovigilance. 2010. Report of CIOMS Working Group VIII.
Guidelines on Good pharmacovigilance practices. 2024.
Regusci A, Lava SAG, Milani GP, Bianchetti MG, Simonetti GD, Vanoni F. Tubulointerstitial nephritis and uveitis syndrome: a systematic review. Nephrol Dialysis Transpl. 2022;37:876–86. https://doi.org/10.1093/ndt/gfab030.
Alaygut D, Bayram MT, Ünlü M, Soylu A, Türkmen M, Kavukçu S. Acute tubulointerstitial nephritis-uveitis (TINU) syndrome developed secondary to paracetamol and codeine phosphate use: two case reports. Turk J Pediatr. 2014;56:92–6.
Venugopal P, Damodaran S, Stocks R A case of tubulointerstitial nephritis and uveitis (TINU) syndrome. Archives of Disease in Childhood. 2012;97: https://doi.org/10.1136/archdischild-2012-301885.389.
Santoro D, Vita G, Rovito S, Venuto L, Cavallari V, Vita R, et al. Drug-induced TINU syndrome and genetic characterization. Clin Nephrol. 2012;78:230–6. https://doi.org/10.5414/cn107119.
Aguilar MC, Lonngi M, de-la-Torre A. Tubulointerstitial Nephritis and Uveitis Syndrome: Case Report and Review of the Literature. Ocul Immunol Inflamm. 2016;24:415–21. https://doi.org/10.3109/09273948.2015.1034374.
Kolomeyer AM, Kodati S. Lamotrigine-Induced Tubulointerstitial Nephritis and Uveitis-Atypical Cogan Syndrome. Eur J Ophthalmol. 2015;26:e14–6. https://doi.org/10.5301/ejo.5000674.
Li JY, Yong TY, Bennett G, Barbara JA, Coates PTH. Human leucocyte antigen DQ alpha heterodimers and human leucocyte antigen DR alleles in tubulointerstitial nephritis and uveitis syndrome. Nephrol (Carlton). 2008;13:755–7. https://doi.org/10.1111/j.1440-1797.2008.00984.x.
Ashraf M, Elhusseiny AM, Kwan JT, Rashad R, Williams E, Marks M, et al. HLA Class I Alleles are strongly associated with lamotrigine-induced SJS/TEN in a US population. Invest Ophthalmol Vis Sci. 2024;65:4234. https://iovs.arvojournals.org/article.aspx?articleid=2798893.
Joyce E, Glasner P, Ranganathan S, Swiatecka-Urban A. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol. 2016;32:577–87. https://doi.org/10.1007/s00467-016-3394-5.
Lee AR, Sharma S, Mahmoud TH. Tubulointerstitial nephritis and uveitis syndrome with a primary presentation of acute posterior multifocal placoid pigment epitheliopathy. Retinal Cases Brief Rep. 2017;11:100–3. https://doi.org/10.1097/ICB.0000000000000299.
Author information
Authors and Affiliations
Contributions
AME was responsible for designing the review protocol. AA conducted the statistical analysis and signal detection analysis, created tables and figures, and updated the reference list. AA and DGD were responsible for writing the manuscript. TKE and AKH helped in writing the manuscript. RHE reviewed the related literature. MZC reviewed the statistical analysis. LA, BRN, PHP reviewed and edited the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
BRN is a member of the Eye I editorial board. No other conflicts.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, A., Downes, D.G., Eleiwa, T.K. et al. Drug-induced tubulointerstitial nephritis and uveitis syndrome in a nationwide surveillance. Eye 39, 2036–2041 (2025). https://doi.org/10.1038/s41433-025-03808-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03808-z
This article is cited by
-
Drug-induced tubulointerstitial nephritis and uveitis syndrome
Reactions Weekly (2025)