Abstract
Objective
To investigate the variations in clinical presentation, imaging characteristics, and immunohistochemical features between benign and malignant orbital lymphoproliferative disorders (OLPDs).
Methods
At the Eye Center of the First Affiliated Hospital, Zhejiang University School of Medicine, a retrospective analysis was performed on the clinical records of all patients diagnosed with OLPDs from 2014 to 2022. This study included a comparative analysis between patients diagnosed with benign and malignant OLPDs.
Results
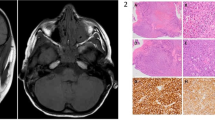
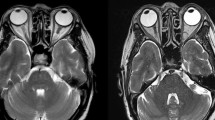
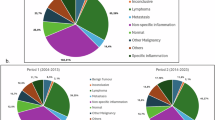
A total of 84 patients were included in this research, with a mean age of 62.24 ± 14.52 years and male-to-female ratio of 43:41. The pathological types comprised benign orbital lymphoproliferative tumours (26/84, 31%, mean age 59.31 ± 10.4 years, male-to-female ratio of 16:10) and malignant orbital lymphomas (58/84, 69%, mean age 63.55 ± 15.93 years, male-to-female ratio of 27:31). The most common symptoms in benign and malignant OLPDs were palpable mass and eyelid swelling. Typically, malignant orbital lymphoma masses exhibited low signal intensity on T1-weighted imaging (T1WI), whereas benign lymphoproliferative tumours commonly demonstrated iso-intensity on T1WI. Notably, the expression of cluster of differentiation 3, 5, 10, 23, 43, and human immunoglobulin light chain lambda demonstrated statistically significant differences between the benign and malignant OLPD groups.
Conclusion
Benign and malignant OLPDs exhibit similar clinical presentations and imaging characteristics. Currently, distinguishing between benign and malignant OLPDs still requires pathological biopsy. Research on specialized imaging techniques and specific biomarkers for malignant orbital lymphomas is underway, showing potential for enhancing the accuracy of non-invasive diagnoses for benign and malignant OLPDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Hu H, Xu X-Q, Liu H, Hong X-N, Shi H-B, Wu F-Y. Orbital benign and malignant lymphoproliferative disorders: Differentiation using semi-quantitative and quantitative analysis of dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol. 2017;88:88–94.
Haradome K, Haradome H, Usui Y, Ueda S, Kwee TC, Saito K, et al. Orbital lymphoproliferative disorders (OLPDs): value of MR imaging for differentiating orbital lymphoma from benign OPLDs. AJNR Am J Neuroradiol. 2014;35:1976–82.
Shields JA, Shields Cl Fau - Scartozzi R, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111:997–1008.
Shinder R, Al-Zubidi N, Fau - Esmaeli B, Esmaeli B. Survey of orbital tumors at a comprehensive cancer center in the United States. Head Neck J Sci Spec. 2011;33:610–4.
Ohtsuka K, Hashimoto M, Fau -, Suzuki Y, Suzuki Y. A review of 244 orbital tumors in Japanese patients during a 21-year period: origins and locations. Jpn J Ophthalmol. 2005;49:49–55.
Ohtsuka K, Hashimoto M, Fau -, Suzuki Y, Suzuki Y. High incidence of orbital malignant lymphoma in Japanese patients. Am J Ophthalmol. 2004;138:881–2.
Olsen TG, Holm F, Mikkelsen LH, Rasmussen PK, Coupland SE, Esmaeli B, et al. Orbital lymphoma-an International multicenter retrospective study. Am J Ophthalmol. 2019;199:44–57.
Ueda S, Usui Y, Nagai T, Diaz-Aguilar D, Nagao T, Goto H. Immunophenotypic profiles for distinguishing orbital mucosa-associated lymphoid tissue lymphoma from benign lymphoproliferative tumors. Jpn J Ophthalmol. 2017;61:354–60.
Briscoe D, Safieh C, Ton Y, Shapiro H, Assia EI, Kidron D. Characteristics of orbital lymphoma: a clinicopathological study of 26 cases. Int Ophthalmol. 2018;38:271–7.
Ahmed OA-O, Ma AK, Ahmed TM, Pointdujour-Lim R. Epidemiology, outcomes, and prognostic factors of orbital lymphoma in the United States. Orbit. 2020;39:397–402.
Hunt SA-O, Pereni I, Ford RA-O, Garrott H. Lymphoma and inflammatory disorders presenting in the orbit- a comparison of characteristics from a 10-year series in a tertiary hospital. Orbit. 2022;41:315–20.
Demirci H, Shields Cl Fau -, Karatza EC, Karatza Ec Fau - Shields JA, Shields JA. Orbital lymphoproliferative tumors: analysis of clinical features and systemic involvement in 160 cases. Ophthalmology. 2008;115:1626–31.
Alaggio RA-O, Amador CA-O, Anagnostopoulos IA-O, Attygalle AA-O, Araujo IBO, Berti EA-O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720–48.
Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol. 2019;64:45–66.
Hsu CR, Chen YY, Yao M, Wei YH, Hsieh YT, Liao SA-O. Orbital and ocular adnexal lymphoma: a review of epidemiology and prognostic factors in Taiwan. Eye. 2021;35:1946–53.
Sagiv O, Thakar SD, Manning JT, Kandl TJ, Fayad LE, Fowler N, et al. Prevalence of a histologic change of ocular adnexal lymphoma in patients with a history of lymphoma. Ophthalmic Plast Reconstr Surg. 2019;35:243–6.
Xu XQ, Hu H, Liu H, Wu JF, Cao P, Shi HB, et al. Benign and malignant orbital lymphoproliferative disorders: Differentiating using multiparametric MRI at 3.0T. J Magn Reson Imaging. 2017;45:167–76.
Priego G, Majos C, Fau -, Climent F, Climent F, Fau, et al. Orbital lymphoma: imaging features and differential diagnosis. Insights Imaging. 2012;3:337–44.
Ren J, Yuan Y, Wu Y, Tao XA-O. Differentiation of orbital lymphoma and idiopathic orbital inflammatory pseudotumor: combined diagnostic value of conventional MRI and histogram analysis of ADC maps. BMC Med Imaging. 2018;18:6.
Akansel G, Hendrix L, Fau - Erickson BA, Erickson Ba Fau - Demirci A, Demirci A, Fau - Papke A, et al. MRI patterns in orbital malignant lymphoma and atypical lymphocytic infiltrates. Eur J Radio. 2005;53:175–81.
Sullivan TJ, Valenzuela AA. Imaging features of ocular adnexal lymphoproliferative disease. Eye. 2006;20:1189–95.
Yeo Jh Fau - Jakobiec FA, Jakobiec Fa Fau - Abbott GF, Abbott Gf Fau - Trokel SL, Trokel SL. Combined clinical and computed tomographic diagnosis of orbital lymphoid tumors. Am J Ophthalmol. 1982;94:235–45.
Polito E, Galieni P, Fau -, Leccisotti A, Leccisotti A. Clinical and radiological presentation of 95 orbital lymphoid tumors. Graefes Arch Clin Exp Ophthalmol. 1996;234:504–9.
Tunlayadechanont P, Panyaping T, Kaewkerd B. Role of quantitative spectral CT analysis for differentiation of orbital lymphoma and other orbital lymphoproliferative disease. Eur J Radio. 2020;133:109372.
Xu XQ, Qian W, Hu H, Su GY, Liu H, Shi HA-O, et al. Histogram analysis of dynamic contrast-enhanced magnetic resonance imaging for differentiating malignant from benign orbital lymphproliferative disorders. Acta Radio. 2019;60:239–46.
Cameron CA, Tong JY, Juniat V, Patel S, Selva D. Diagnostic utility of diffusion-weighted imaging and apparent diffusion coefficient for common orbital lesions: a review. Ophthalmic Plast Reconstr Surg. 2022;38:515–21.
Xie X, Yang L, Zhao FA-O, Wang D, Zhang H, He X, et al. A deep learning model combining multimodal radiomics, clinical and imaging features for differentiating ocular adnexal lymphoma from idiopathic orbital inflammation. Eur Radio. 2022;32:6922–32.
Yang L, Zhang H, Xie X, Jiang S, Zhang H, Cao X, et al. MRI-based radiomics nomogram for preoperative differentiation between ocular adnexal lymphoma and idiopathic orbital inflammation. J Magn Reson Imaging. 2023;57:1594–604.
Shimizu H, Usui Y, Wakita R, Aita Y, Tomita A, Tsubota K, et al. Differential Tissue Metabolic Signatures in IgG4-related ophthalmic disease and orbital mucosa-associated lymphoid tissue lymphoma. Invest Ophthalmol Vis Sci. 2021;62:15.
Nezu N, Usui YA-O, Asakage M, Shimizu H, Tsubota KA-O, Narimatsu A, et al. Distinctive tissue and serum MicroRNA profile of IgG4-related ophthalmic disease and MALT Lymphoma. J Clin Med. 2020;9. https://doi.org/10.3390/jcm9082530 LID–2530.
Shi J, Zhu T, Lin H, Liu Z, Zhou M, Yu Z, et al. Proteotranscriptomics of ocular adnexal B-cell lymphoma reveals an oncogenic role of alternative splicing and identifies a diagnostic marker. J Exp Clin Cancer Res. 2022;41:234.
Acknowledgements
We thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Funding
The work was supported by the Key R&D Program of Zhejiang Province of China (2021C03032), the TCM Innovation Talent Support Program (2023ZR106), and the Special Fund for the Construction of Nursing Discipline of the First Affiliated Hospital of Zhejiang University School of Medicine (2023ZYHL05).
Author information
Authors and Affiliations
Contributions
Xiawei Wang and Hongguang Cui were responsible for designing and writing the protocol. Menghui Zhang, Ying Chen, and Xinyun Wang obtained and analyzed the data. Xiawei Wang and Menghui Zhang drafted the manuscript. Hongguang Cui modified the manuscript for academic content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhang, M., Chen, Y. et al. Characteristics of orbital lymphoproliferative disorders: a retrospective study of 84 cases. Eye 39, 2096–2103 (2025). https://doi.org/10.1038/s41433-025-03816-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03816-z