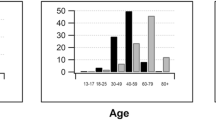
Abstract
Purpose
To summarize the characteristics and methodology of non-inferiority trials in ophthalmology, aiding researchers in understanding the applications and limitations of such trials in ophthalmic diseases.
Methods
PubMed, Web of Science, Embase and Scopus were searched for literature on non-inferiority randomized trials in ophthalmology published between 2000 and November 5 2023. Data on the basic characteristics were extracted and summarized. The Risk of Bias 2’s was used to assess the bias risk.
Results
A total of 294 papers were included, with 77.6% of the trials conducted in the last 10 years, and more than 2/3 (72.1%) were multicenter studies, and 79.9% were registered on platforms. The majority of trials were applied in the researches of glaucoma, cataract, age macular degeneration, macular edema, dry eye, myopia, or refractive error. Non-inferiority thresholds were reported in 88.4% of the trials. Intent-to-treat analysis was the primary outcome analysis method in only 21.8% of trials, while both intent-to-treat and per-protocol analyses were used in 29.6%. Last observation carried forward method was used to address missing values in 23.5%. However, 56.5% of the articles did not report how missing values were handled, leaving uncertainty regarding whether missing data was considered in the analysis. About 20.7% of the studies were at high risk of bias, mainly due to outcome measures and missing value treatments.
Conclusion
Non-inferiority trials are commonly used in ophthalmologic research to assess the effectiveness, safety, cost-effectiveness of treatments or surgical methods, but the quality of implementation and reporting needs to be improved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). C. E 10 choice of control group and related issues in clinical trials-guidance for industry. In:2001. https://www.fda.gov/media/71349/download.
Greene CJ, Morland LA, Durkalski VL, Frueh BC. Noninferiority and equivalence designs: issues and implications for mental health research. J Trauma Stress. 2008;21:433–9.
Kim KS, Chan AW, Belley-Cote EP, Drucker AM. Noninferiority randomized controlled trials. J Invest Dermatol. 2022;142:1773–7.
Hung HM, Wang SJ, O’Neill R. A regulatory perspective on choice of margin and statistical inference issue in non-inferiority trials. Biom J. 2005;47:28–36.
Hernandez AV, Pasupuleti V, Deshpande A, Thota P, Collins JA, Vidal JE. Deficient reporting and interpretation of non-inferiority randomized clinical trials in HIV patients: a systematic review. PLoS One. 2013;8:e63272.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–604.
Aberegg SK, Hersh AM, Samore MH. Empirical Consequences of Current Recommendations for the Design and Interpretation of Noninferiority Trials. J Gen Intern Med. 2018;33:88–96.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Murthy VL, Desai NR, Vora A, Bhatt DL. Increasing proportion of clinical trials using noninferiority end points. Clin Cardiol. 2012;35:522–3.
Suda KJ, Hurley AM, McKibbin T. Motl MS. Publication of noninferiority clinical trials: changes over a 20-year interval. Pharmacotherapy. 2011;31:833–9.
Aupiais C, Zohar S, Taverny G, Le Roux E, Boulkedid R, Alberti C. Exploring how non-inferiority and equivalence are assessed in paediatrics: a systematic review. Arch Dis Child. 2018;103:1067–75.
Kaul S. Understanding the merits and drawbacks of noninferiority trials in cardiovascular medicine. Can J Cardiol. 2021;37:1378–93.
Komorowski AS, Bai AD, Cvetkovic A, Mourad O, Lo CKL, Li XX, et al. Methodological and reporting quality of non-inferiority randomized controlled trials comparing antifungal therapies: a systematic review. Clin Microbiol Infect. 2022;28:640–8.
Li Y, He Y, Sheng Y, Wang K, Wang J, Huang J, et al. Systematic evaluation of non-inferiority and equivalence randomized trials of anti-infective drugs. Expert Rev Anti Infect Ther. 2013;11:1377–89.
Althunian TA, de Boer A, Groenwold R, Klungel OH. Defining the noninferiority margin and analysing noninferiority: an overview. Br J Clin Pharm. 2017;83:1636–42.
Fleming TR, Odem-Davis K, Rothmann MD, Li SY. Some essential considerations in the design and conduct of non-inferiority trials. Clin Trials. 2011;8:432–9.
Scott IA. Non-inferiority trials: determining whether alternative treatments are good enough. Med J Aust. 2009;190:326–30.
Dasgupta A, Lawson KA, Wilson JP. Evaluating equivalence and noninferiority trials. Am J Health Syst Pharm. 2010;67:1337–43.
Gomberg-Maitland M, Frison L, Halperin JL. Active-control clinical trials to establish equivalence or noninferiority: methodological and statistical concepts linked to quality. Am Heart J. 2003;146:398–403.
Piaggio G, Elbourne DR, Altman DG, Evans SJ, Altman DG, CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152–60.
Rehal S, Morris TP, Fielding K, Carpenter JR, Phillips PP. Non-inferiority trials: are they inferior? A systematic review of reporting in major medical journals. BMJ Open. 2016;6:e012594.
Donken R, de Melker HE, Rots NY, Berbers G, Knol MJ. Comparing vaccines: a systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine. 2015;33:1426–32.
Wangge G, Klungel OH, Roes KC, de Boer A, Hoes AW, Knol MJ. Room for improvement in conducting and reporting non-inferiority randomized controlled trials on drugs: a systematic review. PLoS One. 2010;5:e13550.
Ganju J, Rom D. Non-inferiority versus superiority drug claims: the (not so) subtle distinction. Trials. 2017;18:278.
Mauri L, D’Agostino RS. Challenges in the design and interpretation of noninferiority trials. N Engl J Med. 2017;377:1357–67.
Tamayo-Sarver JH, Albert JM, Tamayo-Sarver M, Cydulka RK. Advanced statistics: how to determine whether your intervention is different, at least as effective as, or equivalent: a basic introduction. Acad Emerg Med. 2005;12:536–42.
Hinman RS, Kasza J. Research note: non-inferiority trials. J Physiother. 2023;69:129–32.
Kim K, Zeraatkar D, Pitre TS, Phillips M, Wykoff CC, Garg SJ, et al. Noninferiority randomised trials in ophthalmology. Eye (Lond). 2023;37:3059–60.
Wasserstein RL, Schirm AL, Lazar NA. Moving to a World Beyond “ p<0.05”. Am Stat. 2019;73:1–19.
Brittain E, Lin D. A comparison of intent-to-treat and per-protocol results in antibiotic non-inferiority trials. Stat Med. 2005;24:1–10.
Garrett AD. Therapeutic equivalence: fallacies and falsification. Stat Med. 2003;22:741–62.
Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–5.
Huitfeldt B, Hummel J. The draft FDA guideline on non-inferiority clinical trials: a critical review from European pharmaceutical industry statisticians. Pharm Stat. 2011;10:414–9.
Schmidt-Erfurth U, Waldstein SM, Deak GG, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:822–32.
Hykin P, Prevost AT, Sivaprasad S, Vasconcelos JC, Murphy C, Kelly J, et al. Intravitreal ranibizumab versus aflibercept versus bevacizumab for macular oedema due to central retinal vein occlusion: the LEAVO non-inferiority three-arm RCT. Health Technol Assess. 2021;25:1–196.
Hykin P, Prevost AT, Vasconcelos JC, Murphy C, Kelly J, Ramu J, et al. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol. 2019;137:1256–64.
Chen S, Haziza D. Multiply robust imputation procedures for zero-inflated distributions in surveys. Metron. 2017;75:333–43.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Rabe BA, Day S, Fiero MH, Bell ML. Missing data handling in non-inferiority and equivalence trials: a systematic review. Pharm Stat. 2018;17:477–88.
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. Br J Surg. 2009;96:342–9.
Thomas ET, Heneghan C. Catalogue of bias: selective outcome reporting bias. BMJ Evid Based Med. 2022;27:370–2.
Author information
Authors and Affiliations
Contributions
DLL and JHL conducted the search, selection, data extraction and quality assessment of relevant literature. DLL and JHL contributed to the statistical analysis and wrote the manuscript. XXD, CL, AG and LJZ revised the manuscript. CWP designed the experiment and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Andrzej Grzybowski is a member of the Eye editorial board. The other authors declare that they have no conflict of interest.
Ethics
This review has been prospectively registered with PROSPERO and has been given a registration number (CRD42023468112).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, DL., Liu, JH., Dong, XX. et al. Non-inferiority trials in clinical ophthalmology: a systematic review. Eye 39, 2151–2158 (2025). https://doi.org/10.1038/s41433-025-03819-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03819-w