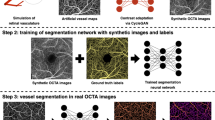
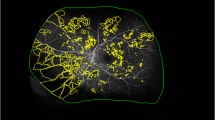
Abstract
Purpose
To demonstrate the capabilities of single-shot widefield swept-source OCT angiography (SS-OCTA) in detecting subclinical retinal neovascularization (RNV), quantifying nonperfusion areas (NPAs), and exploring the relations between NPAs and subclinical RNV in eyes graded as nonproliferative diabetic retinopathy (NPDR).
Methods
Eyes clinically graded as moderate to severe NPDR underwent SS-OCTA imaging. Expert graders identified subclinical RNV, defined as vessels with a flow signal above the internal limiting membrane on OCTA that are not visible on dilated fundus examination. This identification was based on a combination of en face OCT, en face OCTA, and cross-sectional OCTA overlaid on OCT. NPA index was calculated as a percentage of automatically quantified NPA over the area in the posterior pole, the mid-periphery, and the total imaged area.
Results
Totally 37 eyes, including 21 had severe NPDR and 16 had moderate NPDR. Subclinical RNV was present in 14 eyes (37.8%). The eyes with RNV had significantly higher mid-peripheral and total NPA indices but not in the posterior region (mid-peripheral NPA: 31.97% ± 7.02% vs. 24.80% ± 6.60%, p = 0.041; total NPA: 27.96% ± 6.36% vs. 21.61% ± 5.65%, p = 0.046; all values are reported as mean ± standard deviation). The total NPA index showed the highest diagnostic accuracy for subclinical RNV detection (AUC: 0.761; 95% CI, 0.592–929, with a sensitivity of 64.3% and a specificity of 87% at a cutoff value of 28.84%).
Conclusion
Widefield SS-OCTA can detect subclinical RNV. The eyes with higher mid-peripheral NPA indices are more likely to have subclinical RNV, indicating that the NPA index may be a useful biomarker for identifying eyes at risk of RNV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request. Access may be subject to restrictions to protect patient confidentiality and institutional policy.
References
Lundeen EA, Burke-Conte Z, Rein DB, Wittenborn JS, Saaddine J, Lee AY, et al. Prevalence of diabetic retinopathy in the US in 2021. JAMA Ophthalmol. 2023;141:747–54.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Wykoff CC, Yu HJ, Avery RL, Ehlers JP, Tadayoni R, Sadda SR. Retinal non-perfusion in diabetic retinopathy. Eye. 2022;36:249–56.
Silva PS, Dela Cruz AJ, Ledesma MG, van Hemert J, Radwan A, Cavallerano JD, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465–72.
Lim JI, Kim SJ, Bailey ST, Kovach JL, Vemulakonda GA, Ying GS, et al. Diabetic retinopathy preferred practice pattern(R). Ophthalmology. 2025;132:P75–162.
Jia Y, Bailey ST, Hwang TS, McClintic SM, Gao SS, Pennesi ME, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci USA. 2015;112:E2395–402.
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25.
Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134:367–73.
You QS, Guo Y, Wang J, Wei X, Camino A, Zang P, et al. Detection of clinically unsuspected retinal neovascularization with wide-field optical coherence tomography angiography. Retina. 2020;40:891–7.
Tsuboi K, Mazloumi M, Guo Y, Wang J, Flaxel CJ, Bailey ST, et al. Utility of en face OCT for the detection of clinically unsuspected retinal neovascularization in patients with diabetic retinopathy. Ophthalmol Retina. 2023;7:683–91.
Tsuboi K, Mazloumi M, Guo Y, Wang J, Flaxel CJ, Bailey ST, et al. Early sign of retinal neovascularization evolution in diabetic retinopathy: a longitudinal OCT angiography study. Ophthalmol Sci. 2024;4:100382.
Russell JF, Flynn HW Jr., Sridhar J, Townsend JH, Shi Y, Fan KC, et al. Distribution of diabetic neovascularization on ultra-widefield fluorescein angiography and on simulated widefield OCT angiography. Am J Ophthalmol. 2019;207:110–20.
Liang GB, Hormel TT, Wei X, Guo Y, Wang J, Hwang T, et al. Single-shot OCT and OCT angiography for slab-specific detection of diabetic retinopathy. Biomed Opt Express. 2023;14:5682–95.
Guo Y, Hormel TT, Gao L, You Q, Wang B, Flaxel CJ, et al. Quantification of nonperfusion area in montaged widefield OCT angiography using deep learning in diabetic retinopathy. Ophthalmol Sci. 2021;1:100027.
Wang J, Hormel TT, You Q, Guo Y, Wang X, Chen L, et al. Robust non-perfusion area detection in three retinal plexuses using convolutional neural network in OCT angiography. Biomed Opt Express. 2020;11:330–45.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82.
Guo Y, Camino A, Zhang M, Wang J, Huang D, Hwang T, et al. Automated segmentation of retinal layer boundaries and capillary plexuses in wide-field optical coherence tomographic angiography. Biomed Opt Express. 2018;9:4429–42.
Hormel TT, Wang J, Bailey ST, Hwang TS, Huang D, Jia Y. Maximum value projection produces better en face OCT angiograms than mean value projection. Biomed Opt Express. 2018;9:6412–24.
Guo Y, Camino A, Wang J, Huang D, Hwang TS, Jia Y. MEDnet, a neural network for automated detection of avascular area in OCT angiography. Biomed Opt Express. 2018;9:5147–58.
Guo Y, Hormel TT, Xiong H, Wang B, Camino A, Wang J, et al. Development and validation of a deep learning algorithm for distinguishing the nonperfusion area from signal reduction artifacts on OCT angiography. Biomed Opt Express. 2019;10:3257–68.
Guo Y, Hormel TT, Gao M, You Q, Wang J, Flaxel CJ, et al. Multi-plexus nonperfusion area segmentation in widefield OCT angiography using a deep convolutional neural network. Transl Vis Sci Technol. 2024;13:15.
Choudhry N, Duker JS, Freund KB, Kiss S, Querques G, Rosen R, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3:843–9.
Nicholson L, Ramu J, Chan EW, Bainbridge JW, Hykin PG, Talks SJ, et al. Retinal nonperfusion characteristics on ultra-widefield angiography in eyes with severe nonproliferative diabetic retinopathy and proliferative diabetic retinopathy. JAMA Ophthalmol. 2019;137:626–31.
Yu G, Aaberg MT, Patel TP, Iyengar RS, Powell C, Tran A, et al. Quantification of retinal nonperfusion and neovascularization with ultrawidefield fluorescein angiography in patients with diabetes and associated characteristics of advanced disease. JAMA Ophthalmol. 2020;138:680–8.
Nakao S, Yoshida S, Kaizu Y, Yamaguchi M, Wada I, Ishibashi T, et al. Microaneurysm detection in diabetic retinopathy using OCT angiography may depend on intramicroaneurysmal turbulence. Ophthalmol Retina. 2018;2:1171–3.
Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y. Automated quantification of nonperfusion in three retinal plexuses using projection-resolved optical coherence tomography angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:5101–6.
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201.
You QS, Wang J, Guo Y, Pi S, Flaxel CJ, Bailey ST, et al. Optical Coherence tomography angiography avascular area association with 1-year treatment requirement and disease progression in diabetic retinopathy. Am J Ophthalmol. 2020;217:268–77.
Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–12.
Wang F, Saraf SS, Zhang Q, Wang RK, Rezaei KA. Ultra-widefield protocol enhances automated classification of diabetic retinopathy severity with OCT angiography. Ophthalmol Retina. 2020;4:415–24.
Nicholson L, Vazquez-Alfageme C, Ramu J, Triantafyllopoulou I, Patrao NV, Muwas M, et al. Validation of concentric rings method as a topographic measure of retinal nonperfusion in ultra-widefield fluorescein angiography. Am J Ophthalmol. 2015;160:1217–25.e2.
Quinn N, Csincsik L, Flynn E, Curcio CA, Kiss S, Sadda SR, et al. The clinical relevance of visualising the peripheral retina. Prog Retin Eye Res. 2019;68:83–109.
Stino H, Huber KL, Niederleithner M, Mahnert N, Sedova A, Schlegl T, et al. Association of diabetic lesions and retinal nonperfusion using widefield multimodal imaging. Ophthalmol Retina. 2023;7:1042–50.
Rand LI, Prud’homme GJ, Ederer F, Canner PL. Factors influencing the development of visual loss in advanced diabetic retinopathy. Diabetic Retinopathy Study (DRS) Report No. 10. Investig Ophthalmol Vis Sci. 1985;26:983–91.
Tan CS, Chew MC, van Hemert J, Singer MA, Bell D, Sadda SR. Measuring the precise area of peripheral retinal non-perfusion using ultra-widefield imaging and its correlation with the ischaemic index. Br J Ophthalmol. 2016;100:235–9.
Mehta N, Cheng Y, Alibhai AY, Duker JS, Wang RK, Waheed NK. Optical coherence tomography angiography distortion correction in widefield montage images. Quant Imaging Med Surg. 2021;11:928–38.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: The International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–22.
Funding
This work was supported by grants from National Institutes of Health (R01 EY 036429, R01 EY035410, R01 EY024544, R01 EY027833, R01 EY031394, R43EY036781, P30 EY010572, T32 EY023211, UL1TR002369); the Jennie P. Weeks Endowed Fund; the Malcolm M. Marquis, MD Endowed Fund for Innovation; Unrestricted Departmental Funding Grant and Dr. H. James and Carole Free Catalyst Award from Research to Prevent Blindness (New York, NY); Edward N. & Della L. Thome Memorial Foundation Award, and the Bright Focus Foundation (G2020168, M20230081). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
AW designed the methodology, collected data, conducted analysis, interpreted results, and drafted the original manuscript. YG contributed to data review, analysis, and result interpretation. TTH provided critical manuscript revisions. CJF, MT, STB, and DP assisted with data collection, offered clinical insights, and reviewed and edited the manuscript. YJ provided expertise in OCTA, contributed to study design and data interpretation, and critically revised the manuscript. TSH conceptualized the study, supervised the research process, interpreted results, and provided critical revisions and final approval. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
YG: Visionix/Optovue (P), Genentech (R), Ifocus Imaging (P); TTH: Ifocus Imaging (I); STB: Visionix/Optovue (F); YJ: Visionix/Optovue (P, R), Roche/Genentech (P, R, F), Ifocus Imaging (I, P), Optos (P), Boeringer Ingelheim (C), Kugler (R). These potential conflicts of interest have been reviewed and managed by OHSU. Other authors declare no conflicts of interest related to this article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, AL., Guo, Y., Hormel, T.T. et al. Wide-field OCTA quantified peripheral nonperfusion areas predict the risk of subclinical neovascularization. Eye 39, 2467–2473 (2025). https://doi.org/10.1038/s41433-025-03891-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03891-2