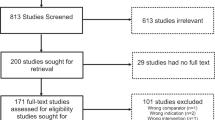
Abstract
Purpose
The purpose of this study was to evaluate the association between glycaemic status and the incidence of third, fourth, and sixth cranial nerve palsy (CNP).
Methods
This is a retrospective nationwide population-based cohort study using South Korean National Health Insurance Service (NHIS) data since 2009. Health check-up data of 4,067,842 individuals aged from 20 to 90 years in the period from 1 January 2009 to 31 December 2018 were analysed. The subjects were classified according to glycaemic status as follows: non-diabetes, impaired fasting glucose (IFG), newly detected diabetes, diabetes duration <5 years, and diabetes duration ≥5 years. The primary endpoint of this study was the incidence of third, fourth or sixth CNP. Hazard ratio (HR) and 95% confidence interval (CI) of CNP were estimated using Cox proportional hazards regression analysis. In Model 5, we adjusted for age, gender, smoking status, alcohol consumption, physical activity, body mass index, hypertension, dyslipidaemia, and chronic kidney disease.
Results
During the follow-up period (mean, 6.3 years), 5835 cases of third, fourth, or sixth CNP were identified with 4,062,007 control cases. In the adjusted model 5, the adjusted HR for third, fourth, and sixth CNP in the IFG group was 1.098 (95% CI 1.030 - 1.171); in the newly detected diabetes group, 1.779 (95% CI 1.587–1.994); in the diabetes duration <5 years group, 1.921 (95% CI 1.731–2.131); and in the diabetes duration ≥5 years group, 2.571 (95% CI 2.343–2.820). Using the Kaplan-Meier curve, the log-rank test demonstrated an increase in the incidence of CNP proportional to the duration of diabetes (p < 0.001).
Conclusions
This large-scale, population-based cohort study suggests that the risk of third, fourth, and sixth CNP significantly increased in patients with IFG and diabetes compared to those with normal glycaemic status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
09 December 2025
The original online version of this article was revised: The article “The relationship between glycaemic status and the risk of third, fourth and sixth cranial nerve palsy: a nationwide population-based study (2009–2018)”, written by Chaeyeon Lee, Kyung-Do Han, Juhwan Yoo et al, was originally published open access under a “Creative Commons Attribution (CC BY) license 4.0”. As a result of the author’s/authors' subsequent decision not to publish this article under the open access model, the copyright notice of the article was changed on 19 November 2025 to © The Author(s), under exclusive licence to The Royal College of Ophthalmologists 2025 with all rights reserved.
19 December 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41433-025-04160-y
References
Dosunmu EO, Hatt SR, Leske DA, Hodge DO, Holmes JM. Incidence and etiology of presumed fourth cranial nerve palsy: a population-based study. Am J Ophthalmol. 2018;185:110–4.
Fang C, Leavitt JA, Hodge DO, Holmes JM, Mohney BG, Chen JJ. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23–8.
Jung EH, Kim SJ, Lee JY, Cho BJ. The incidence and etiology of sixth cranial nerve palsy in Koreans: A 10-year nationwide cohort study. Sci Rep. 2019;9:18419.
Jung EH, Kim SJ, Lee JY, Cho BJ. The incidence and etiologies of third cranial nerve palsy in Koreans: A 10-year nationwide cohort study. Ophthalmic Epidemiol. 2020;27:460–7.
Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004;111:369–75.
Tamhankar MA, Biousse V, Ying GS, Prasad S, Subramanian PS, Lee MS, et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120:2264–9.
Bendszus M, Beck A, Koltzenburg M, Vince GH, Brechtelsbauer D, Littan T, et al. MRI in isolated sixth nerve palsies. Neuroradiology. 2001;43:742–5.
Akagi T, Miyamoto K, Kashii S, Yoshimura N. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol. 2008;52:32–5.
Chou KL, Galetta SL, Liu GT, Volpe NJ, Bennett JL, Asbury AK, et al. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci. 2004;219:35–9.
Murchison AP, Gilbert ME, Savino PJ. Neuroimaging and acute ocular motor mononeuropathies: a prospective study. Arch Ophthalmol. 2011;129:301–5.
Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol. 1994;112:961–6.
Sanders SK, Kawasaki A, Purvin VA. Long-term prognosis in patients with vasculopathic sixth nerve palsy. Am J Ophthalmol. 2002;134:81–4.
Watanabe K, Hagura R, Akanuma Y, Takasu T, Kajinuma H, Kuzuya N, et al. Characteristics of cranial nerve palsies in diabetic patients. Diab Res Clin Pr. 1990;10:19–27.
Greco D, Gambina F, Pisciotta M, Abrignani M, Maggio F. Clinical characteristics and associated comorbidities in diabetic patients with cranial nerve palsies. J Endocrinol Invest. 2012;35:146–9.
Kobashi R, Ohtsuki H, Hasebe S. Clinical studies of ocular motility disturbances: Part 2. Risk factors for ischemic ocular motor nerve palsy [corrected]. Jpn J Ophthalmol. 1997;41:115–9.
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diab Care. 2010;33:2285–93.
El Mansouri Y, Zaghloul K, Amraoui A. Oculomotor paralyses in the course of diabetes-concerning 12 cases. J Fr Ophtalmol. 2000;23:14–8.
Al Kahtani ES, Khandekar R, Al-Rubeaan K, Youssef AM, Ibrahim HM, Al-Sharqawi AH. Assessment of the prevalence and risk factors of ophthalmoplegia among diabetic patients in a large national diabetes registry cohort. BMC Ophthalmol. 2016;16:118.
Nanayakkara N, Ranasinha S, Gadowski A, Heritier S, Flack JR, Wischer N, et al. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J Diab Complications. 2018;32:279–90.
Bansal N. Prediabetes diagnosis and treatment: A review. World J Diab. 2015;6:296–303.
World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia : report of a WHO/IDF consultation Geneva, Switzerland: World Health Organization; 2006.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diab Care. 2014;37:S81–90.
Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640.
Hollander P, Spellman C. Controversies in prediabetes: do we have a diagnosis?. Postgrad Med. 2012;124:109–18.
Twigg SM, Kamp MC, Davis TM, Neylon EK, Flack JR, Australian Diabetes Society, et al. Prediabetes: a position statement from the Australian Diabetes Society and Australian Diabetes Educators Association. Med J Aust. 2007;186:461–5.
Buysschaert M, Bergman M. Definition of prediabetes. Med Clin North Am. 2011;95:289–97.
Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–90.
Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953.
Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–44.
Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med. 2011;171:1352–60.
Mahat RK, Singh N, Arora M, Rathore V. Health risks and interventions in prediabetes: A review. Diab Metab Syndr. 2019;13:2803–11.
Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39:1377–84.
Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: Physiopathology and treatment. World J Diab. 2015;6:432–44.
van Dam PS. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diab Metab Res Rev. 2002;18:176–84.
Dejgaard A. Pathophysiology and treatment of diabetic neuropathy. Diabet Med. 1998;15:97–112.
Greco D, Gambina F, Maggio F. Ophthalmoplegia in diabetes mellitus: a retrospective study. Acta Diabetol. 2009;46:23–6.
Aaberg ML, Burch DM, Hud ZR, Zacharias MP. Gender differences in the onset of diabetic neuropathy. J Diab Complications. 2008;22:83–7.
Patel SV, Holmes JM, Hodge DO, Burke JP. Diabetes and hypertension in isolated sixth nerve palsy: a population-based study. Ophthalmology. 2005;112:760–3.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2021R1A2C1007718).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The article “The relationship between glycaemic status and the risk of third, fourth and sixth cranial nerve palsy: a nationwide population-based study (2009–2018)”, written by Chaeyeon Lee, Kyung-Do Han, Juhwan Yoo et al, was originally published open access under a “Creative Commons Attribution (CC BY) license 4.0”. As a result of the author’s/authors' subsequent decision not to publish this article under the open access model, the copyright notice of the article was changed on 19 November 2025 to © The Author(s), under exclusive licence to The Royal College of Ophthalmologists 2025 with all rights reserved.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, C., Han, KD., Yoo, J. et al. The relationship between glycaemic status and the risk of third, fourth and sixth cranial nerve palsy: a nationwide population-based study (2009–2018). Eye 40, 185–191 (2026). https://doi.org/10.1038/s41433-025-04011-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-04011-w