Abstract
Background
To explore the potential mechanisms of myopia by detecting metabolites in the aqueous humour (AH) of young patients with myopia without fundus complications.
Methods
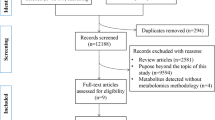
Randomised controlled trials (RCTs). A total of 65 AH samples (65 eyes) were collected in Weifang Eye Hospital. Untargeted metabolomics was conducted via liquid chromatography‒tandem mass spectrometry (LC‒MS/MS). Receiver operating characteristic (ROC) curves were generated.
Results
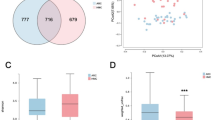
Patients were divided into three groups according to axial length (AL). There were 21 patients (21 eyes) in the moderate-myopia (MM) group (24.00 mm < AL ≤ 26.00 mm), 24 patients (24 eyes) in the high-myopia (HM) group (26.00 mm < AL ≤ 28.00 mm), and 20 patients (20 eyes) in the ultrahigh-myopia (UHM) group (AL > 28.00 mm). A total of 2203 metabolites were detected in the AH samples, and there were 62 shared differentially abundant metabolites among the three groups. KEGG pathways among the three groups included sphingolipid metabolism, lipid and atherosclerosis, and the pentose phosphate pathway (PPP). The key metabolites DL-glutamine, phytosphingosine, D-erythrose 4-phosphate, and pyridoxine phosphate exhibited the strongest correlations with AL (all P values were <0.0001). In the ROC analysis, DL-glutamine, phytosphingosine, and D-erythrose 4-phosphate were good biomarkers for MM. Pyridoxine phosphate was a potential biomarker for UHM.
Conclusions
Abnormal sphingolipid metabolism was closely related to myopia. Changes in phytosphingosine levels in the AH among various AL groups may serve as early warning signs of retinal changes. These metabolic pathways and metabolites could serve as intervention targets for myopia management in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated and analysed during the current study are available from the corresponding author upon reasonable request.
References
Jonas JB, Jonas RA, Bikbov MM, Wang YX, Panda-Jonas S. Myopia: Histology, clinical features, and potential implications for the etiology of axial elongation. Progr Retinal Eye Res. 2023;96:101156.
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42.
Jong M, Naduvilath T, Saw J, Kim K, Flitcroft DI. Association between Global Myopia Prevalence and International Levels of Education. Optom Vis Sci. 2023;100:702–7.
Dong L, Kang YK, Li Y, Wei WB, Jonas JB. Prevalence and time trends of myopia in children and adolescents in CHINA a systemic review and meta-analysis. Retin-J Ret Vit Dis. 2020;40:399–411.
Zhang XY, Zhou YL, Wang Y, Du W, Yang J. Trend of myopia through different interventions from 2010 to 2050: Findings from Eastern Chinese student surveillance study. Front Med-Lausanne. 2023;9:1069649.
Matsumura S, Kuo AN, Saw SM. An update of eye shape and myopia. Eye Contact Lens. 2019;45:279–85.
Lee JTL, Guo XX, Li ZX, Jong M, Sankaridurg P, He MG. Progression and longitudinal biometric changes in highly myopic eyes. Invest Ophth Vis Sci. 2020;61:34.
Wang YY, Chen SS, Lin J, Chen W, Huang HM, Fan X, et al. Vascular changes of the choroid and their correlations with visual acuity in pathological myopia. Invest Ophth Vis Sci. 2022;63:20.
Jiang F, Wang DC, Yin QX, He MG, Li ZX. Longitudinal changes in axial length and spherical equivalent in children and adolescents with high myopia. Invest Ophth Vis Sci. 2023;64:6.
Shi HK, Guo NJ, Zhao ZM, He XY, Li JH, Duan JL. Global prevalence of myopic macular degeneration in general population and patients with high myopia: a systematic review and meta-analysis. Eur J Ophthalmol. 2024;34:631–40.
Yu C, Xu CC, Wang ZC, Zhang XH, Huang XM. Color doppler ultrasound analysis of pathological myopia induced changes in retrobulbar blood flow and its relationship with characteristic changes in myopia. Pak J Med Sci. 2023;39:853–7.
Hayashi K, Manabe S, Hirata A, Yoshimura K. Posterior vitreous detachment in highly myopic patients. Invest Ophth Vis Sci. 2020;61:33.
Jonas JB, Wang YX, Dong L, Panda-Jonas S. High myopia and glaucoma-like optic neuropathy. Asia-Pac J Ophthalmo. 2020;9:234–8.
Liao CM, Ding XH, Han XT, Jiang Y, Zhang J, Scheetz JN, et al. Role of parental refractive status in myopia progression: 12-year annual observation from the guangzhou twin eye study. Invest Ophth Vis Sci. 2019;60:3499–506.
Yu MK, Hu YY, Han M, Song JW, Wu ZY, Xu ZH, et al. Global risk factor analysis of myopia onset in children: a systematic review and meta-analysis. Plos One. 2023;18:e0291470.
Grochowski ET, Pietrowska K, Kowalczyk T, Mariak Z, Kretowski A, Ciborowski M, et al. Omics in myopia. J Clin Med. 2020;9:3464.
Du B, Jin N, Zhu XR, Lu DQ, Jin CC, Li Z, et al. A prospective study of serum metabolomic and lipidomic changes in myopic children and adolescents. Experimental Eye Res. 2020;199:108182.
Teo AWJ, Zhang JW, Zhou L, Liu YC. Metabolomics in corneal diseases: a narrative review from clinical aspects. Metabolites. 2023;13:380.
Liu K, Fang JW, Jin J, Zhu SP, Xu XY, Xu YP, et al. Serum metabolomics reveals personalized metabolic patterns for macular neovascular disease patient stratification. J Proteome Res. 2020;19:699–707.
Chen XL, Chen YH, Wang L, Sun XH. Metabolomics of the aqueous humor in patients with primary congenital glaucoma. Mol Vis. 2019;25:489–501.
Midena E, Frizziero L, Midena G, Pilotto E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graef Arch Clin Exp. 2021;259:3549–60.
Costagliola C. dell’Omo R, Agnifili L, Bartollino S, Fea AM, Uva MG, et al. How many aqueous humor outflow pathways are there? Surv Ophthalmol. 2020;65:144–70.
Hou XW, Wang Y, Ke CF, Pan CW. Metabolomics facilitates the discovery of metabolic profiles and pathways for myopia: a systematic review. Eye. 2023;37:670–7.
Lian P, Zhao XJ, Song HY, Tanumiharjo S, Chen J, Wang T, et al. Metabolic characterization of human intraocular fluid in patients with pathological myopia. Experimental Eye Res. 2022;222:109184.
Yue Y, Che D, Hsiao YW, Zhou J, Zhao K. Association between transforming growth factors -β and matrix metalloproteinases in the aqueous humor and plasma in myopic patients. J Francais D Ophtalmologie. 2022;45:1177–83.
Tang YP, Zhang XB, Hu ZX, Lin K, Lin Z, Chen TY, et al. Vitreous metabolomic signatures of pathological myopia with complications. Eye. 2023;37:2987–93.
Nenni M, Çelebier M, Maçin S, Örsten S, Çiftçi SY, Baysal I. Untargeted metabolomics to discriminate liver and lung hydatid cysts: Importance of metabolites involved in the immune response. Vet Parasitol. 2024;328:110180.
Jonas JB, Xu L. Histological changes of high axial myopia. Eye. 2014;28:113–7.
Nazifova-Tasinova N, Radev M, Galunska B, Grupcheva C. Metabolomic analysis in ophthalmology. Biomed Pap. 2020;164:236–46.
Ji YH, Rao J, Rong XF, Lou S, Zheng Z, Lu Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp Eye Res. 2017;159:147–55.
Barbas-Bernardos C, Armitage EG, García A, Mérida S, Navea A, Bosch-Morell F, et al. Looking into aqueous humor through metabolomics spectacles - exploring its metabolic characteristics in relation to myopia. J Pharm Biomed. 2016;127:18–25.
Zhu XJ, Du Y, Truscott RJW, He WW, Zhoug P, Lu Y. Profiling and Bioinformatic Analysis of Differentially Expressed Cytokines in Aqueous Humor of High Myopic Eyes - Clues for Anti-VEGF Injections. Curr Eye Res. 2020;45:97–103.
Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diab Complicat. 2019;33:195–201.
Shiwani HA, Elfaki MY, Memon D, Ali S, Aziz A, Egom EE. Updates on sphingolipids: spotlight on retinopathy. Biomed Pharmacother. 2021;143:112197.
AL Qtaish N, Gallego I, Beitia IV, Sainz-Ramos M, Martínez-Navarrete G, Soto-Sanchez C, et al. Sphingolipid extracts enhance gene delivery of cationic lipid vesicles into retina and brain. Eur J Pharm Biopharm. 2021;169:103–12.
Mondal K, Mandal N. Role of bioactive sphingolipids in inflammation and eye diseases. Role Bioact Lipids Cancer Inflamm Relat Dis. 2019;1161:149–67.
Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019;18:e13048.
Liu B, Cong CY, Li ZE, Hao LL, Yuan XM, Wang WQ, et al. Analysis of the aqueous humor lipid profile in patients with polypoidal choroidal vasculopathy. Experimental Eye Res. 2022;222:109160.
Che DLL, Cao Y, Zhang Y, Yu Q, Li F, Zhou J. Lipid profile in the aqueous humor of patients with myopia. Exp Eye Res. 2024;8:110023.
Teslaa T, Ralser M, Fan J, Rabinowitz JD. The pentose phosphate pathway in health and disease. Nat Metab. 2023;5:1275–89.
Nadaban A, Frame CO, El Yachioui D, Gooris GS, Dalgliesh RM, Malfois M, et al. The sphingosine and phytosphingosine ceramide ratio in lipid models forming the short periodicity phase: an experimental and molecular simulation study. Langmuir. 2024;40:13794–809.
Presa N, Gomez-Larrauri A, Rivera IG, Ordoñez M, Trueba M, Gomez-Muñoz A. Regulation of cell migration and inflammation by ceramide 1-phosphate. Bba-Mol Cell Biol L. 2016;1861:402–9.
Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Bba-Biomembranes. 2006;1758:1864–84.
Zhao JN, Tan Y, Wang L, Su X, Shi Y. Serum sphingosine-1-phosphate levels and gene polymorphisms in acute respiratory distress syndrome: a multicenter prospective study (Retracted article. See vol. 20, 2022). J Transl Med. 2020;18:156.
Grambergs R, Mondal K, Mandal N. Inflammatory ocular diseases and sphingolipid signaling. Bioact Ceramides Health Dis: Intertwined Roles Enigmatic Lipids. 2019;1159:139–52.
Barbier T, Collard F, Zúñiga-Ripa A, Moriyón I, Godard T, Becker J, et al. Erythritol feeds the pentose phosphate pathway via three new isomerases leading to D-erythrose-4-phosphate in. Proc Natl Acad Sci USA. 2014;111:17815–20.
Guerriero RM, Patel AA, Walsh B, Baumer FM, Shah AS, Peters JM, et al. Systemic manifestations in pyridox(am)ine 5′-phosphate oxidase deficiency. Pediatr Neurol. 2017;76:47–53.
Nee J, Jörres A, Krannich A, Leithner C, Schroeder T, Munk AL, et al. Elimination of glutamate using CRRT for 72 h in patients with post-cardiac arrest syndrome: a randomized clinical pilot trial. Resuscitation. 2019;144:54–9.
Saw SM, Matsumura S, Hoang QV. Prevention and management of myopia and myopic pathology. Invest Ophth Vis Sci. 2019;60:488–99.
Russo A, Boldini A, Romano D, Mazza G, Bignotti S, Morescalchi F, et al. Myopia: mechanisms and strategies to slow down its progression. J Ophthalmol. 2022;2022:1004977.
Funding
This work was supported by the National Natural Science Foundation of China [grant no. 82271114]; the Natural Science Foundation of Zhejiang Province, China [grant no. LZ22H120001 and ZCLTGD24H1201]; Science and Technology Development Plan Project of Weifang, Shandong Province, China [grant no. 2023YX070 and 2025ZJ1121].
Author information
Authors and Affiliations
Contributions
MF and XZ: conducted the experiments and wrote the manuscript. XS and YX: statistical analysis and figures. JZ, JW, YW, and KW: ICL preoperation and postoperation examinations and data collection. HZ and QT: ICL surgery and AH collection. Z-LC: designed the study and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Weifang Eye Hospital (approval number: 2023-01-03) and was in compliance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, M., Zhang, X., Song, X. et al. Metabolomic characteristics of aqueous humor in young myopia patients without fundus complications. Eye 40, 348–355 (2026). https://doi.org/10.1038/s41433-025-04168-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-04168-4