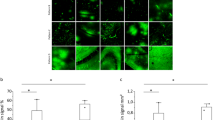
Abstract
Transcranial Magnetic Resonance Guided Focused Ultrasound can oscillate intravenously delivered microbubbles and transiently open the blood brain barrier (BBB) in a targeted brain region. However, high microbubble doses or Focused ultrasound pressures (FUS) leads to injury. So, we administered nitrous oxide (N2O), an anesthetic gas to determine reduced need of FUS pressure and microbubble dose for opening BBB. Swiss Webster mice were treated with N2O or medical air (MA) at varying FUS pressures, while the microbubble dose was kept constant and the vice-versa. Consequently, BBB opening was quantified by acoustic emissions and enhancement rate on T1-weighted MR. To compare the effect of N2O on gene delivery, following BBB opening with either MA or N2O, a viral vector expressing GFP was subsequently delivered. Additionally, Immunohistochemical studies quantified viral transfection efficacy and assessed acute cell injury. We observed that N2O significantly potentiates acoustic emissions and enhancement rate on post-contrast MRI images, compared to MA at all measured pressures (0.39, 0.45, 0.67 MPa). Furthermore, N2O reduces the microbubble dose to 0.02μl/kg and FUS pressures to 0.28 and 0.39 MPa for BBB disruption and enhanced viral gene delivery, respectively. Hence, N2O potentiates microbubble oscillations, allowing reduced microbubble dose and FUS pressures and improved viral gene delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data discussed here will be made available to readers upon request.
References
Fenstermacher J, Gazendam J. Intra-arterial infusions of drugs and hyperosmotic solutions as ways of enhancing CNS chemotherapy. Cancer Treat Rep. 1981;65:27–37.
Rapoport SI, Hori M, Klatzo I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am J Physiol. 1972;223:323–31.
Hersh DS, Wadajkar AS, Roberts N, Perez JG, Connolly NP, Frenkel V, et al. Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des. 2016;22:1177–93.
Pardridge W. M. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18:157–67.
Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–104.
Bing C, Ladouceur-Wodzak M, Wanner CR, Shelton JM, Richardson JA, Chopra R. Trans-cranial opening of the blood-brain barrier in targeted regions using a stereotaxic brain atlas and focused ultrasound energy. J Ther Ultrasound. 2014;2:13.
Cheng B, Bing C, Xi Y, Shah B, Exner AA, Chopra R. Influence of Nanobubble Concentration on Blood-Brain Barrier Opening Using Focused Ultrasound Under Real-Time Acoustic Feedback Control. Ultrasound Med Biol. 2019;45:2174–87.
McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–37.
Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008;55:124–31.
Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N. Safety Validation of repeated blood-brain barrier disruption using focused ultrasound. Ultrasound Med Biol. 2016;42:481–92.
Samiotaki G, Vlachos F, Tung YS, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med. 2012;67:769–77.
Chien C,Y, Xu L, Pacia C,P, Yue Y, Chen H. Blood–brain barrier opening in a large animal model using closed-loop microbubble cavitation-based feedback control of focused ultrasound sonication. Sci Rep. 2022;12:16147.
Tsai CH, Zhang JW, Liao YY, Liu HL. Real-time monitoring of focused ultrasound blood-brain barrier opening via subharmonic acoustic emission detection: implementation of confocal dual-frequency piezoelectric transducers. Phys Med Biol. 2016;61:2926–46.
Tung YS, Marquet F, Teichert T, Ferrera V, Konofagou EE. Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Appl Phys Lett. 2011;98:163704.
Ku MC, Waiczies S, Niendorf T, Pohlmann A. Assessment of blood brain barrier leakage with gadolinium-enhanced MRI. Methods Mol Biol. 2018;1718:395–408.
Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS ONE. 2012;7:e45783.
Bader KB, Holland CK. Gauging the likelihood of stable cavitation from ultrasound contrast agents. Phys Med Biol. 2013;58:127–44.
McDannold N, Zhang Y, Vykhodtseva N. Blood-brain barrier disruption and vascular damage induced by ultrasound bursts combined with microbubbles can be influenced by choice of anesthesia protocol. Ultrasound Med Biol. 2011;37:259–1270.
McDannold N, Zhang Y, Supko JG, Power C, Sun T, Peng C, et al. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics. 2019;9:6284–99.
Sassaroli E, Hynynen K. Resonance frequency of microbubbles in small blood vessels: a numerical study. Phys Med Biol. 2005;50:5293–305.
Qamar A, Samtaney R, Bull JL. Dynamics of micro-bubble sonication inside a phantom vessel. Appl Phys Lett. 2013;102:13702.
Chen C, Gu Y, Tu J, Guo X, Zhang D. Microbubble oscillating in a microvessel filled with viscous fluid: A finite element modeling study. Ultrasonics. 2016;66:54–64.
Cipolla MJ. In: The cerebral circulation. San Rafael, CA: Morgan & Claypool Life Sciences; 2009.
McDannold N, Zhang Y, Vykhodtseva N. The effects of oxygen on ultrasound-induced blood-brain barrier disruption in mice. Ultrasound Med Biol. 2017;43:469–75.
Mullin L, Gessner R, Kwan J, Kaya M, Borden MA, Dayton PA. Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media Mol Imaging. 2011;6:126–31.
Hosseinkhah N, Hynynen K. A three-dimensional model of an ultrasound contrast agent gas bubble and its mechanical effects on microvessels. Phys Med Biol. 2012;57:785–808.
Lapin NA, Gill K, Shah BR, Chopra R. Consistent opening of the blood brain barrier using focused ultrasound with constant intravenous infusion of microbubble agent. Sci Rep. 2020;10:16546.
Lockwood AJ, Yang YF. Nitrous oxide inhalation anaesthesia in the presence of intraocular gas can cause irreversible blindness. Br Dent J. 2008;204:247–8.
Bailey RM, Rozenberg A, Gray SJ. Comparison of high-dose intracisterna magna and lumbar puncture intrathecal delivery of AAV9 in mice to treat neuropathies. Brain Res. 2020;1739:146832.
Gray SJ, Choi VW, Asokan A, Haberman RA, McCown TJ, Samulski RJ. Production of recombinant adeno-associated viral vectors and use in in vitro and in vivo administration. Curr Protoc Neurosci. 2006;4:17.
El-Sharkawey A. Calculate the corrected total cell fluorescence (CTCF). 2016. https://doi.org/10.13140/RG.2.1.1307.8008.
Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J Cereb Blood Flow Metab. 2011;31:725–37.
Samiotaki G, Konofagou EE. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:2257–65.
Bing C, Hong Y, Hernandez C, Rich M, Cheng B, Munaweera I, et al. Characterization of different bubble formulations for blood-brain barrier opening using a focused ultrasound system with acoustic feedback control. Sci Rep. 2018;8:7986.
Tung YS, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol. 2010;55:6141–55.
Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. 2019;10: 4373.
Tung YS, Vlachos F, Feshitan JA, Borden MA, Konofagou EE. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am. 2011;130:3059–67.
Meng Y, MacIntosh BJ, Shirzadi Z, Kiss A, Bethune A, Heyn C, et al. Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood-brain barrier opening in patients with Alzheimer’s disease. Neuroimage. 2019;200:275–80.
Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. 2020;10:1–9.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76.
van Liew HD, Burkard ME. Behavior of bubbles of slowly permeating gas used for ultrasonic imaging contrast. Investig Radiol. 1995;30:315–21.
Benavides R, Maze M, Franks NP. Expansion of gas bubbles by nitrous oxide and xenon. Anesthesiology. 2006;104:299–302.
Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune responses to viral gene therapy vectors. Mol Ther. 2020;28:709–22.
Downs ME, Buch A, Sierra C, Karakatsani ME, Teichert T, Chen S, et al. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE. 2015;10:e0125911.
Choi HJ, Han M, Seo H, Park CY, Lee EH, Park J. The new insight into the inflammatory response following focused ultrasound-mediated blood–brain barrier disruption. Fluids Barriers CNS. 2022;19:103.
Funding
UTSW High Impact award: BRS, RMB, RC.
Author information
Authors and Affiliations
Contributions
BRS designed the work, performed experiments, prepared and revised manuscript; DB and IY performed experiments, manuscript preparation, and statistical analysis; DI manuscript preparation; VK, SKH, SJET, RC, RMB performed experiments and results interpretation; MD did manuscript preparation. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
RC has competing interests with FUS Instruments. Other authors declare no competing interests.
Ethical approval
The animal protocol (101517) for this study was approved by the ethics committee of University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee (IACUC) and Confirmed to the National Institutes of Health’s PHS Policy on the Humane Care and Use of Laboratory Animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhardwaj, D., Youssef, I., Imphean, D. et al. Nitrous oxide enhances MR-guided focused ultrasound delivery of gene therapy to the murine hippocampus. Gene Ther 32, 376–384 (2025). https://doi.org/10.1038/s41434-025-00530-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41434-025-00530-z