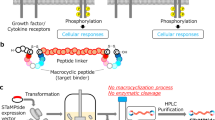
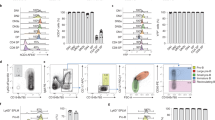
Abstract
Chimeric peptides hold promising potential to be introduced as an ideal gene delivery platform based on their advantages over viral carriers, including but not limited to the safety profile and specific targeting. However, their gene transfer efficiency needs improvement. Here, we designed a new multi-functional chimeric peptide for enhanced gene delivery by adding a cyclic TAT motif to a previously designed MPG2H peptide to enable the targeting of cells with independent/dependent endocytosis cell entry mechanisms. CTATMPG2H was expressed and purified using affinity chromatography; then it was characterized through a gel retardation assay, circular dichroism (CD) spectropolarimetry, transmission electron microscopy (TEM) dynamic light scattering (DLS), and zeta potential analysis. CTATMPG2H was compared with MiRGD as a chimeric peptide control in all steps. After assessing the platform stability in various conditions, its gene transfer efficiency was evaluated in the HEK293T cell line with reporter genes. Additionally, mouse bone marrow-derived dendritic cells (BMDCs) were transfected to test CTATMPG2H potential in immunotherapy. The results illustrated a safe gene transfer profile for CTATMPG2H comparable to MiRGD and Polyethyleneimine (PEI). Flow cytometry results showed up to 48% gene transfer rate for CTATMPG2H to dendritic cells with minimal toxicity (viability rate ~80%). Moreover, the in silico investigation showed that the synergistic effects of electrostatic, hydrogen, and hydrophobic interactions enhance the stability and binding affinity of peptide-pDNA complexes, ensuring robust and specific targeting of nucleic acids. This research sets a foundation for future in vivo studies and potential clinical applications, aiming for safer and more effective gene therapy strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Raw Data is also provided in supplementary Information. Some parts of the datasets generated and/or analyzed during the current study are not publicly available due to the process of Patent Filling, but are available from the corresponding author on reasonable request.
References
Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–8.
Miller AD. Human gene therapy comes of age. Nature. 1992;357:455–60.
Nayerossadat N, Ali P, Maedeh T. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27.
Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95.
Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, et al. Dendritic cells as pharmacological tools for cancer immunotherapys. Pharmacol Rev. 2015;67:731–53.
Alycia G, Brian R. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–65.
Mahato RI, Monera OD, Smith LC, Rolland A. Peptide-based gene delivery. Curr Opin Mol Ther. 1999;1:226–43.
Canine BF, Hatefi A. Development of recombinant cationic polymers for gene therapy research. Adv Drug Deliv Rev. 2010;62:1524–9.
Cheraghi R, Nazari M, Alipour M, Hosseinkhani S. Stepwise development of biomimetic chimeric peptides for gene delivery. Protein Pept Lett. 2020;27:698–710.
Rusiecka, I, Gągało, I & Kocić, I Cell-penetrating peptides improve pharmacokinetics and pharmacodynamics of anticancer drugs. Tissue Barriers 2022;10:1965418.
Zhang X, Lei T, Du H. Prospect of cell penetrating peptides in stem cell tracking. Stem Cell Res Ther. 2021;12:457.
Sadeghian I, Heidari R, Sadeghian S, Raee MJ, Negahdaripour M. Potential of cell-penetrating peptides (CPPs) in delivery of antiviral therapeutics and vaccines. Eur J Pharm Sci. 2022;169:106094.
Zhang Z, Baxter AE, Ren D, Qin K, Chen Z, Collins SM et al. Efficient engineering of human and mouse primary cells using peptide-assisted genome editing. Nat Biotechnol. 2023. https://doi.org/10.1038/s41587-023-01756-1.
Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–6.
Deshayes S, Morris M, Heitz F, Divita G. Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Adv Drug Deliv Rev. 2008;60:537–47.
Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: Implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31:2717–24.
Sadeghian F, Hosseinkhani S, Alizadeh A, Hatefi A. Design, engineering and preparation of a multi-domain fusion vector for gene delivery. Int J Pharm. 2012;427:393–9.
Majidi A, Nikkhah M, Sadeghian F, Hosseinkhani S. Development of novel recombinant biomimetic chimeric MPG-based peptide as nanocarriers for gene delivery: Imitation of a real cargo. Eur J Pharm Biopharm. 2016;107:191–204.
Cheraghi R, Nazari M, Alipour M, Majidi A, Hosseinkhani S. Development of a Targeted anti-HER2 scFv Chimeric Peptide for Gene Delivery into HER2-Positive Breast Cancer Cells. Int J Pharm. 2016;515:632–43.
Alipour M, Majidi A, Molaabasi F, Sheikhnejad R, Hosseinkhani S. In vivo tumor gene delivery using novel peptideticles: pH-responsive and ligand targeted core–shell nanoassembly. Int J Cancer. 2018;143:2017–28.
Alipour M, Hosseinkhani S, Sheikhnejad R, Cheraghi R. Nano-biomimetic carriers are implicated in mechanistic evaluation of intracellular gene delivery. Sci Rep. 2017;7:41507.
Zohrabi T, Hosseinkhani S. Ternary nanocomplexes of metallic nanoclusters and recombinant peptides for fluorescence imaging and enhanced gene delivery. Mol Biotechnol. 2020;62:495–507.
Golestanipour A, Nikkhah M, Aalami A, Hosseinkhani S. Gene delivery to tobacco root cells with single-walled carbon nanotubes and cell-penetrating fusogenic peptides. Mol Biotechnol. 2018;60:863–78.
Ghafary SM, Nikkhah M, Hatamie S, Hosseinkhani S. Simultaneous gene delivery and tracking through preparation of photo-luminescent nanoparticles based on graphene quantum dots and chimeric peptides. Sci Rep. 2017;7:9552.
Moasses Ghafary S, Rahimjazi E, Hamzehil H, Modarres Mousavi M, Nikkhah M, Hosseinkhani S. et al. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots. Nanomed Nanotechnol Biol Med. 2022;42.
Alipour M, Sheikhnejad R, Fouani MH, Bardania H, Hosseinkhani S. DNAi-peptide nanohybrid smart particles target BCL-2 oncogene and induce apoptosis in breast cancer cells. Biomed Pharmacother. 2023;166.
Wang H, Feng Z, Xu B. Supramolecular assemblies of peptides or nucleopeptides for gene delivery. Theranostics. 2019;9:3213–22.
Lehto T, Ezzat K, Wood MJA, Andaloussi EL. S. Peptides for nucleic acid delivery. Adv Drug Deliv Rev. 2016;106:172–82.
Thapa RK, Sullivan MO. Gene delivery by peptide-assisted transport. Curr Opin Biomed Eng. 2018;7:71–82.
Hoyer J, Neundorf I. Peptide vectors for the nonviral delivery of nucleic acids. Acc Chem Res. 2012;45:1048–56.
Wang X, Tai Z, Tian J, Zhang W, Yao C, Zhang L, et al. Reducible chimeric polypeptide consisting of octa-D-arginine and tetra-L-histidine peptides as an efficient gene delivery vector. Int J Nanomed. 2015;10:4669–90.
Salomone F, Cardarelli F, Signore G, Boccardi C, Beltram F. In vitro efficient transfection by CM18-Tat11 hybrid peptide: a new tool for gene-delivery applications. PLoS ONE 2013;8:e70108.
Teo SLY, Rennick JJ, Yuen D, Al-Wassiti H, Johnston APR, Pouton CW. Unravelling cytosolic delivery of cell penetrating peptides with a quantitative endosomal escape assay. Nat Commun. 2021;12:3721.
Sahni A, Qian Z, Pei D. Cell-penetrating peptides escape the endosome by inducing vesicle budding and collapse. ACS Chem Biol. 2020;15:2485–92.
Zhao Y, Jiang H, Yu J, Wang L, Du J. Engineered histidine-rich peptides enhance endosomal escape for antibody-targeted intracellular delivery of functional proteins. Angew Chemie Int Ed Engl. 2023;62:e202304692.
Khamehchian S, Nikkhah M, Madani R, Hosseinkhani S. Enhanced and selective permeability of gold nanoparticles functionalized with cell penetrating peptide derived from maurocalcine animal toxin. J Biomed Mater Res Part A. 2016;104:2693–2700.
Patel SG, Sayers EJ, He L, Narayan R, Williams TL, Mills EM, et al. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci Rep 2019;9:6298.
Sajid MI, Moazzam M, Stueber R, Park SE, Cho Y, Malik NUA, et al. Applications of amphipathic and cationic cyclic cell-penetrating peptides: significant therapeutic delivery tool. Peptides 2021;141:170542.
Tsuchiya K, Gimenez-Dejoz J, Numata K. Molecular dynamics simulation of complexation between plasmid DNA and cationic peptides. Polym J. 2023;55:1109–14.
Reichelt P, Schwarz C, Donzeau M. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr Purif. 2006;46:483–8.
Cheraghi R, Alipour M, Nazari M, Hosseinkhani S. Optimization of conditions for gene delivery system based on PEI. Mol Pharm. 2017;4:8–16.
Yu B, Pettitt BM, Iwahara J. Dynamics of ionic interactions at protein-nucleic acid interfaces. Acc Chem Res. 2020;53:1802–10.
Solovyev AY, Tarnovskaya SI, Chernova IA, Shataeva LK, Skorik YA. The interaction of amino acids, peptides, and proteins with DNA. Int J Biol Macromol. 2015;78:39–45.
Faltejsková K, Jakubec D, Vondrášek J. Hydrophobic amino acids as universal elements of protein-induced DNA structure deformation. Int J Mol Sci 2020;21:3986.
Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:494–500.
Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–17.
Eiríksdóttir E, Konate K, Langel Ü, Divita G, Deshayes S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim Biophys Acta - Biomembr. 2010;1798:1119–28.
Veldhoen S, Laufer SD, Trampe A, Restle T. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: Quantitative analysis of uptake and biological effect. Nucleic Acids Res. 2006;34:6561–73.
Yoo JW, Doshi N, Mitragotri S. Adaptive micro and nanoparticles: Temporal control over carrier properties to facilitate drug delivery. Adv Drug Deliv Rev. 2011;63:1247–56.
Pappalardo JS, Langellotti CA, Giacomo SD, Olivera V, Quattrocchi V, Zamorano P, et al. In vitro transfection of bone marrow-derived dendritic cells with TATp-liposomes. Int J Nanomed. 2014;9:963–73.
Haines AMR, Irvine AS, Mountain A, Charlesworth J, Farrow NA, Husain RD, et al. CL22 - A novel cationic peptide for efficient transfection of mammalian cells. Gene Ther. 2001;8:99–110.
Tan PH, Beutelspacher SC, Wang Y, McClure MO, Ritter MA, Lombardi G, et al. Immunolipoplexes: An efficient, nonviral alternative for transfection of human dendritic cells with potential for clinical vaccination. Mol Ther. 2005;11:790–800.
Acknowledgements
This work was supported by the National Institute for Medical Research Development (NIMAD) (grant number 400369).
Funding
This work was supported by the National Institute for Medical Research Development (NIMAD) (grant number 400369).
Author information
Authors and Affiliations
Contributions
M.D conducted all the wet lab experiments, prepared the manuscript and figures. S.R conducted in silico investigations and prepared related figures. S.H supervised the project. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All protocols and procedures in the animal study and all experiments described were approved by the Research Ethics Committee of the Faculty of Biological Science – Tarbiat Modares University (Approval ID: IR.MODARES.REC.1400.313) and National Institute for Medical Research Development (Approval ID: IR.NIMAD.REC.1400.177) which was in accordance to the ethical principles and the national norms and standards for conducting medical research in Iran.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dehshiri, M., Rezaei, S. & Hosseinkhani, S. A novel multi-functional chimeric peptide for enhanced safe gene delivery in immunotherapy. Gene Ther 32, 497–507 (2025). https://doi.org/10.1038/s41434-025-00538-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41434-025-00538-5