Abstract
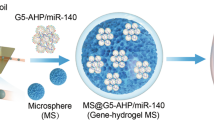
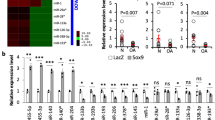
Due to the poor healing capacity of tendons, the healing process is slow, with a risk of re-rupture post-injury. In this study, we found that miR-494-3p was one of the miRNAs with significant expression differences after tendon injury by sequencing in the rat Achilles tendon injury model. Therefore, we hypothesized that regulating miR-494-3p expression in tendons could improve tendon healing. Considering the long healing process of the tendons and the short half-life of miRNA, we hope to achieve the best efficacy by delivering miR-494-3p using a sustained-release nanoparticle hydrogel system. In the results, with an increase in miR-494-3p, the tendon biomechanics were significantly improved after 2-week repair, and the content of collagen I (Col I) also increased. Through bioinformatics prediction, double luciferase, and immunohistochemistry experiments, we confirmed that miR-494-3p targeting CXXC finger protein 4 (CXXC4) promoted tendon healing. In conclusion, the miR-494-3p/nanoparticles hydrogel delivery system can protect and sustainedly transfer miR-494-3p into tenocytes, block the translation of CXXC4, increase the expression of Col I, and ultimately improve tendon healing.

A nanoparticle hydrogel delivery system of miRNA was constructed and applied to injured tendons. Finally, we confirmed that the miR-494-3p/nanoparticles hydrogel delivery system can protect and sustainedly transfer miR-494-3p into tenocytes, block the translation of CXXC4, increase the expression of Col I, and ultimately improve tendon healing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra). Additional raw data are available from the corresponding author upon reasonable request.
References
Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202.
Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: development, repair, regeneration, and healing. J Orthop Res. 2015;33:780–4.
de Jong JP, Nguyen JT, Sonnema AJ, Nguyen EC, Amadio PC, Moran SL. The incidence of acute traumatic tendon injuries in the hand and wrist: a 10-year population-based study. Clin Orthop Surg. 2014;6:196–202.
James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–12.
Cui H, He Y, Chen S, Zhang D, Yu Y, Fan C. Macrophage-Derived miRNA-Containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Mol Ther Nucleic Acids. 2019;14:114–30.
Tang JB. Tendon surgery. Shanghai Science and Technology Press, Shanghai; 2015.
Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. 2015;6:6774.
Dubin JA, Greenberg DR, Iglinski-Benjamin KC, Abrams GD. Effect of micro-RNA on tenocytes and tendon-related gene expression: a systematic review. J Orthop Res. 2018;36:2823–9.
Usman MA, Nakasa T, Shoji T, Kato T, Kawanishi Y, Hamanishi M, et al. The effect of administration of double stranded MicroRNA-210 on acceleration of Achilles tendon healing in a rat model. J Orthop Sci. 2015;20:538–46.
Chen L, Wang GD, Liu JP, Wang HS, Liu XM, Wang Q, et al. miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone. 2015;71:210–6.
Lee SWL, Paoletti C, Campisi M, Osaki T, Adriani G, Kamm RD, et al. MicroRNA delivery through nanoparticles. J Control Release. 2019;313:80–95.
Zhou YL, Yang QQ, Yan YY, Zhu C, Zhang L, Tang JB. Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions. Acta Biomater. 2018;70:237–48.
Sun J, Ju F, Jin J, Wang HL, Li ZJ, Sun YC, et al. M2 macrophage membrane-mediated biomimetic-nanoparticle carrying COX-siRNA targeted delivery for prevention of tendon adhesions by inhibiting inflammation. Small. 2023;19:e2300326.
Xing SG, Zhou YL, Yang QQ, Ju F, Zhang L, Tang JB. Effects of nanoparticle-mediated growth factor gene transfer to the injured microenvironment on the tendon-to-bone healing strength. Biomater Sci. 2020;8:6611–24.
Yang QQ, Zhang L, Zhou YL, Tang JB. Morphological changes of macrophages and their potential contribution to tendon healing. Colloids Surf B Biointerfaces. 2022;209:112145.
Zhou Y, Zhu C, Wu YF, Zhang L, Tang JB. Effective modulation of transforming growth factor-β1 expression through engineered microRNA-based plasmid-loaded nanospheres. Cytotherapy. 2015;17:320–9.
Hu Y, Zhao Z, Ehrich M, Fuhrman K, Zhang C. In vitro controlled release of antigen in dendritic cells using pH-sensitive liposome-polymeric hybrid nanoparticles. Polymer. 2015;80:171–9.
Yang YY, Chia HH, Chung TS. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J Control Release. 2000;69:81–96.
Zhao Z, Hu Y, Hoerle R, Devine M, Raleigh M, Pentel P, et al. A nanoparticle-based nicotine vaccine and the influence of particle size on its immunogenicity and efficacy. Nanomedicine. 2017;13:443–54.
Stoll C, John T, Conrad C, Lohan A, Hondke S, Ertel W, et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials. 2011;32:4806–15.
Zhao Q, Xiong Y, Xu J, Chen S, Li P, Huang Y, et al. Host MicroRNA hsa-miR-494-3p Promotes EV71 replication by directly targeting PTEN. Front Cell Infect Microbiol. 2018;8:278.
Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–82.
Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012.
Guan B, Zhan Z, Wang L, Wang L, Liu L. CXXC4 mediates glucose-induced β-cell proliferation. Acta Diabetol. 2020;57:1101–9.
Kojima T, Shimazui T, Hinotsu S, Joraku A, Oikawa T, Kawai K, et al. Decreased expression of CXXC4 promotes a malignant phenotype in renal cell carcinoma by activating Wnt signaling. Oncogene. 2009;28:297–305.
Liu Y, Xu J, Xu L, Wu T, Sun Y, Lee YW, et al. Cystic fibrosis transmembrane conductance regulator mediates tenogenic differentiation of tendon-derived stem cells and tendon repair: accelerating tendon injury healing by intervening in its downstream signaling.FASEB J.2017;31:3800–15.
Yao Z, Li J, Xiong H, Cui H, Ning J, Wang S, et al. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J Nanobiotechnol. 2021;19:169.
Caldas C, Brenton JD. Sizing up miRNAs as cancer genes. Nat Med. 2005;11:712–4.
Xia W, Zhou J, Luo H, Liu Y, Peng C, Zheng W, et al. MicroRNA-32 promotes cell proliferation, migration and suppresses apoptosis in breast cancer cells by targeting FBXW7. Cancer Cell Int. 2017;17:14.
Iwaki J, Kikuchi K, Mizuguchi Y, Kawahigashi Y, Yoshida H, Uchida E, et al. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS ONE. 2013;8:e69496.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Zhang Y, Guo L, Li Y, Feng GH, Teng F, Li W, et al. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol Cancer. 2018;17:1.
Romano G, Acunzo M, Garofalo M, Di Leva G, Cascione L, Zanca C, et al. MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced apoptosis in non-small-cell lung cancer through BIM down-regulation. Proc Natl Acad Sci USA. 2012;109:16570–5.
Huang W, Lin M, Yang C, Wang F, Zhang M, Gao J, et al. Rat bone mesenchymal stem cell-derived exosomes loaded with miR-494 promoting neurofilament regeneration and behavioral function recovery after spinal cord injury. Oxid Med Cell Longev. 2021;2021:1634917.
Zhan MN, Yu XT, Tang J, Zhou CX, Wang CL, Yin QQ, et al. MicroRNA-494 inhibits breast cancer progression by directly targeting PAK1. Cell Death Dis. 2017;8:e2529.
Kim WK, Park M, Kim YK, Tae YK, Yang HK, Lee JM, et al. MicroRNA-494 downregulates KIT and inhibits gastrointestinal stromal tumor cell proliferation. Clin Cancer Res. 2011;17:7584–94.
Li J, Chen J, Wang S, Li P, Zheng C, Zhou X, et al. Blockage of transferred exosome-shuttled miR-494 inhibits melanoma growth and metastasis. J Cell Physiol. 2019;234:15763–74.
Zhou Y, Zhang L, Zhao W, Wu Y, Zhu C, Yang Y. Nanoparticle-mediated delivery of TGF-β1 miRNA plasmid for preventing flexor tendon adhesion formation. Biomaterials. 2013;34:8269–78.
Langston Suen WL, Chau Y. Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells. J Pharm Pharmacol. 2014;66:564–73.
Gilam A, Conde J, Weissglas-Volkov D, Oliva N, Friedman E, Artzi N, et al. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat Commun. 2016;7:12868.
Fu Y, Wang Z, Luo C, Wang Y, Wang Y, Zhong X, et al. Downregulation of CXXC Finger Protein 4 Leads to a Tamoxifen-resistant phenotype in breast cancer cells through activation of the Wnt/β-catenin Pathway. Transl Oncol. 2020;13:423–40.
Chu M, Sun Z, Fan Z, Yu D, Mao Y, Guo Y. Bi-directional regulation functions of lanthanum-substituted layered double hydroxide nanohybrid scaffolds via activating osteogenesis and inhibiting osteoclastogenesis for osteoporotic bone regeneration. Theranostics. 2021;11:6717–34.
Sun Z, Jin H, Zhou H, Yu L, Wan H, He Y. Guhong Injection promotes fracture healing by activating Wnt/beta-catenin signaling pathway in vivo and in vitro. Biomed Pharmacother. 2019;120:109436.
Dufour AM, Borowczyk-Michalowska J, Alvarez M, Truchetet ME, Modarressi A, Brembilla NC, et al. IL-17A Dissociates inflammation from fibrogenesis in systemic sclerosis. J Investig Dermatol. 2020;140:103–12.e108.
Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. an in vivo study. J Bone Joint Surg Am. 2008;90:1078–89.
Funding
This study was supported by the National Natural Science Foundation of China (Nos 82102186) and the Jiangsu Provincial Medical Key Discipline (XKTJ-XK202003). This study was also supported by the Jiangsu Provincial Medical Innovation Center of Neuromicroscopy and Minimally Invasive Translational Medicine (CXZX202212) and CNNC Youth Talent Program.
Author information
Authors and Affiliations
Contributions
G.H.W. performed most of the experiments, wrote and revised the manuscript. L.W. partly designed and performed the experiments. L.S. partly designed the experiments and revised the manuscript. H.J.S. partly performed the experiments and revised the manuscript. W.G.Z. and Y.L.C. partly performed the experiments and data analysis. A.D.D. and J.T. partly designed and supervised the experiments. X.Z.Z. provided the initial idea of this study, designed the experiments, and revised the manuscript. G.H.W., L.W. and L.S. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments involving Sprague Dawley rats were conducted in accordance with the relevant guidelines and regulations, and were approved by the Experimental Animal Care and Use Committee of Nantong University (Approval number: S20200314-016). All rats were provided by the Laboratory Animal Center of Nantong University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G.H., Wang, L., Sheng, L. et al. Nanoparticle hydrogel system delivery of miR-494-3p to improve tendon healing by targeting CXXC4. Gene Ther (2025). https://doi.org/10.1038/s41434-025-00543-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41434-025-00543-8