Abstract
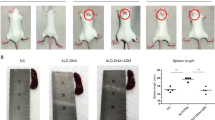
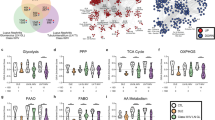
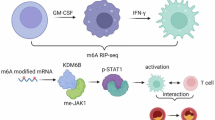
Genetic variants of NCF1 that impair the production of reactive oxygen species (ROS) are associated with lupus in humans; however, the underlying mechanism of immune dysregulation remains unclear. To clarify this mechanism, the study tested the hypothesis that retrotransposons contribute to the early onset of lupus by facilitating the expansion and activation of macrophages. Using the ROS-deficient lupus-prone lpr mouse model, we employed bulk RNA sequencing, flow cytometry, and spatially resolved single-cell transcriptome imaging to comprehensively characterize tissue-resident macrophages. The results demonstrated increased expression of the mouse transcript family type D (MTD) retrotransposon in tissue-resident macrophages from the spleen, kidneys, and skull dura of ROS-deficient lpr mice, indicating a link between ROS deficiency, MTD expression, and macrophage expansion. Importantly, this MTD expression decreased following two weeks of mycophenolate mofetil therapy, linking therapy response to retrotransposon activity. Furthermore, the MTD-encoded RNA was used to disrupt the signaling of retrotransposons, leading to regulatory T-cell activation and downregulation of both glomerular macrophage infiltration and serum interleukin-6 secretion in lupus-prone mice. Collectively, these findings suggest that the MTD retrotransposons play a crucial role in driving the early onset of lupus by enhancing macrophage activation, which in turn promotes immune dysregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information file].
References
Zhong J, Olsson LM, Urbonaviciute V, Yang M, Bäckdahl L, Holmdahl R. Association of NOX2 subunits genetic variants with autoimmune diseases. Free Radic Biol Med. 2018;125:72–80.
Gordon RA, Cosgrove HA, Marinov A, Gingras S, Tilstra JS, Campbell AM, et al. NADPH oxidase in B cells and macrophages protects against murine lupus by regulation of TLR7. JCI Insight. 2024;9:e178563.
Al-Azab M, Idiiatullina E, Liu Z, Lin M, Hrovat-Schaale K, Xian H, et al. Genetic variants in UNC93B1 predispose to childhood-onset systemic lupus erythematosus. Nat Immunol. 2024;25:969–80.
Li Y, Coelho A, Li Z, Alsved M, Li Q, Xu R, et al. The systemic lupus erythematosus-associated NCF190H allele synergizes with viral infection to cause mouse lupus but also limits virus spread. Nat Commun. 2025;16:1593.
Hawtin S, André C, Collignon-Zipfel G, Appenzeller S, Bannert B, Baumgartner L, et al. Preclinical characterization of the Toll-like receptor 7/8 antagonist MHV370 for lupus therapy. Cell Rep Med. 2023;4:101036.
Pasquesi GIM, Allen H, Ivancevic A, Barbachano-Guerrero A, Joyner O, Guo K, et al. Regulation of human interferon signaling by transposon exonization. Cell. 2024;187:7621–36.e19.
Sareila O, Jaakkola N, Olofsson P, Kelkka T, Holmdahl R. Identification of a region in p47phox/NCF1 crucial for phagocytic NADPH oxidase (NOX2) activation. J Leukoc Biol. 2012;93:427–35.
Luo H, Urbonaviciute V, Saei AA, Lyu H, Gaetani M, Végvári Á, et al. NCF1-dependent production of ROS protects against lupus by regulating plasmacytoid dendritic cell development and functions. JCI Insight. 2023;8:e164875.
Parodis I, Lindblom J, Barturen G, Ortega-Castro R, Cervera R, Pers J-O, et al. Molecular characterisation of lupus low disease activity state (LLDAS) and DORIS remission by whole-blood transcriptome-based pathways in a pan-European systemic lupus erythematosus cohort. Ann Rheum Dis. 2024;83:889–900.
Tighe PJ, Stevens SE, Dempsey S, Le Deist F, Rieux-Laucat F, Edgar JDM. Inactivation of the Fas gene by Alu insertion: retrotransposition in an intron causing splicing variation and autoimmune lymphoproliferative syndrome. Genes Immun. 2002;3:S66–S70.
Wu J, Xie F, Qian K, Gibson AW, Edberg JC, Kimberly RP. FAS mRNA editing in human systemic lupus erythematosus. Hum Mutat. 2011;32:1268–77.
Bogutz AB, Brind’Amour J, Kobayashi H, Jensen KN, Nakabayashi K, Imai H, et al. Evolution of imprinting via lineage-specific insertion of retroviral promoters. Nat Commun. 2019;10:5674.
Matsubara H, Shimizu Y, Arai M, Yamagata A, Ito S, Imakiire T, et al. PEPITEM/Cadherin 15 Axis Inhibits T Lymphocyte Infiltration and Glomerulonephritis in a Mouse Model of Systemic Lupus Erythematosus. J Immunol. 2020;204:2043–52.
Sethi S, Madden B, Debiec H, Morelle J, Charlesworth MC, Gross L, et al. Protocadherin 7–Associated Membranous Nephropathy. J Am Soc Nephrol. 2021;32:1249–61.
Van Der Vlist M, Ramos MIP, Van Den Hoogen LL, Hiddingh S, Timmerman LM, De Hond TAP, et al. Signaling by the inhibitory receptor CD200R is rewired by type I interferon. Sci Signal. 2021;14:eabb4324.
Huang C, Zhan L, Hannigan MO, Ai Y, Leto TL. P47phox-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and dbl+. J Leukoc Biol. 2000;67:210–5.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Storer J, Hubley R, Rosen J, Wheeler TJ, Smit AF. The Dfam community resource of transposable element families, sequence models, and genome annotations. Mob DNA. 2021;12:2.
Mann M, Wright PR, Backofen R. IntaRNA 2.0: enhanced and customizable prediction of RNA–RNA interactions. Nucleic Acids Res. 2017;45:W435–W439.
Zhong J, Scholz T, Yau ACY, Guerard S, Hüffmeier U, Burkhardt H, et al. Mannan-induced Nos2 in macrophages enhances IL-17–driven psoriatic arthritis by innate lymphocytes. Sci Adv. 2018;4:eaas9864.
Zhong J, Li Q, Holmdahl R. Natural loss-of-function mutations in Qa2 and NCF1 cause the spread of Mannan-induced psoriasis. J Invest Dermatol. 2021;141:1765–71.e4.
Zhong J, Zheng C, Chen Z, Yue H, Gao H, Jiang Y, et al. Phosphopeptides P140 cause oxidative burst responses of pulmonary macrophages in an imiquimod-induced lupus model. Mol Biomed. 2023;4:38.
Stringer C, Wang T, Michaelos M, Pachitariu M. Cellpose: a generalist algorithm for cellular segmentation. Nat Methods. 2021;18:100–6.
Stirling DR, Swain-Bowden MJ, Lucas AM, Carpenter AE, Cimini BA, Goodman A. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinforma. 2021;22:433.
Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat Nanotechnol. 2020;15:313–20.
Borm, Mossi LE, Albiach A, Mannens CCA, Janusauskas J, Özgün C, et al. Scalable in situ single-cell profiling by electrophoretic capture of mRNA using EEL FISH. Nat Biotechnol. 2023;41:222–31.
Zeng H, Huang J, Ren J, Wang CK, Tang Z, Zhou H, et al. Spatially resolved single-cell translatomics at molecular resolution. Science. 2023;380:eadd3067.
Gorbunova V, Seluanov A, Mita P, McKerrow W, Fenyö D, Boeke JD, et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021;596:43–53.
Perez AA, Goronzy IN, Blanco MR, Yeh BT, Guo JK, Lopes CS, et al. ChIP-DIP maps binding of hundreds of proteins to DNA simultaneously and identifies diverse gene regulatory elements. Nat Genet. 2024;56:2827–41.
Zheng X, Dozmorov MG, Strohlein CE, Bastacky S, Sawalha AH. Ezh2 knockout in B cells impairs plasmablast differentiation and ameliorates lupus-like disease in MRL / lpr mice. Arthritis Rheum. 2023;75:1395–406.
Rauch E, Amendt T, Lopez Krol A, Lang FB, Linse V, Hohmann M, et al. T-bet+ B cells are activated by and control endogenous retroviruses through TLR-dependent mechanisms. Nat Commun. 2024;15:1229.
Baeyens A, Bracero S, Chaluvadi VS, Khodadadi-Jamayran A, Cammer M, Schwab SR. Monocyte-derived S1P in the lymph node regulates immune responses. Nature. 2021;592:290–5.
Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–7.
Zhong J, Yau ACY, Holmdahl R. Regulation of T Cell Function by Reactive Nitrogen and Oxygen Species in Collagen-Induced Arthritis. Antioxid Redox Signal. 2020;32:161–72.
Zhong J, Yau ACY, Holmdahl R. Independent and inter-dependent immunoregulatory effects of NCF1 and NOS2 in experimental autoimmune encephalomyelitis. J Neuroinflamm. 2020;17:113.
Li Q, Zhong J, Luo H, Urbonaviciute V, Xu Z, He C, et al. Two major genes associated with autoimmune arthritis, Ncf1 and Fcgr2b, additively protect mice by strengthening T cell tolerance. Cell Mol Life Sci. 2022;79:482.
Khmaladze I, Kelkka T, Guerard S, Wing K, Pizzolla A, Saxena A, et al. Mannan induces ROS-regulated, IL-17A–dependent psoriasis arthritis-like disease in mice. Proc Natl Acad Sci USA. 2014;111:E3669–E36678.
Zhu W, Lönnblom E, Förster M, Johannesson M, Tao P, Meng L, et al. Natural polymorphism of Ym1 regulates pneumonitis through alternative activation of macrophages. Sci Adv. 2020;6:eaba9337.
Kraaij MD, Savage NDL, Van Der Kooij SW, Koekkoek K, Wang J, Van Den Berg JM, et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:17686–91.
Raeber ME, Caspar DP, Zurbuchen Y, Guo N, Schmid J, Michler J, et al. Interleukin-2 immunotherapy reveals human regulatory T cell subsets with distinct functional and tissue-homing characteristics. Immunity. 2024;57:2232–50.e10.
Chandy KG, DeCoursey TE, Fischbach M, Talal N, Cahalan MD, Gupta S. Altered K + channel expression in abnormal T lymphocytes from mice with the lpr gene mutation. Science. 1986;233:1197–200.
Groß CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, et al. K + Efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45:761–73.
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–7.
Wu J, Raman A, Coffey NJ, Sheng X, Wahba J, Seasock MJ, et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J Clin Invest. 2021;131:e136329.
Abedini A, Levinsohn J, Klötzer KA, Dumoulin B, Ma Z, Frederick J, et al. Single-cell multi-omic and spatial profiling of human kidneys implicates the fibrotic microenvironment in kidney disease progression. Nat Genet. 2024;56:1712–24.
Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, et al. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med. 2017;214:3207–17.
Reynolds J, Huang M, Li Y, Meineck M, Moeckel T, Weinmann-Menke J, et al. Constitutive knockout of interleukin-6 ameliorates memory deficits and entorhinal astrocytosis in the MRL/lpr mouse model of neuropsychiatric lupus. J Neuroinflamm. 2024;21:89.
Zhong J, Tian J, Yang X, Qin C. Whole-body Cerenkov luminescence tomography with the finite element SP3 method. Ann Biomed Eng. 2011;39:1728–35.
Zhong J, Zheng C, Gao H, Tong W, Hui H, Tian J. Noninvasive imaging of the lung NETosis by anti-Ly6G iron oxide nanoparticles. Heliyon. 2022;8:e10043.
Acknowledgements
This work was supported in part by the Beijing Natural Science Foundation [IS23113, L222141, 7252286, J2023010], the National Natural Science Foundation of China [62027901], and the Fundamental Research Funds for the Central Universities [YWF23YG-QB015].
Author information
Authors and Affiliations
Contributions
JZ designed and supervised the study, analyzed the data, and wrote the manuscript. ZC performed the experiments, analyzed the data, and revised the paper. HY, ZH, and WZ performed the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The animal study protocols were approved by the ethical committees of Beihang University, China (BM20210060). All animal models and experimental procedures were performed in accordance with standard laboratory guidelines, institutional biosafety regulations, and manufacturer’s recommendations. No primary human tissues, human subjects, or cell lines were involved in this research.
Consent for publication
Not applicable—this study does not involve human participants and contains no individual participant data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, J., Chen, Z., Yue, H. et al. Macrophage retrotransposon expression is associated with lupus. Genes Immun (2025). https://doi.org/10.1038/s41435-025-00369-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41435-025-00369-9