Abstract
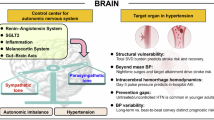
The pathogenesis of hypertension is multifactorial and highly complex. Basic research plays critical roles in elucidating the complex pathogenesis of hypertension and developing its treatment. This review covers recent topics in basic research related to hypertension in the following six parts: brain/autonomic nervous system, kidney, vascular system, potential treatments, extracellular vesicles, and gut microbiota. The brain receives afferent nerve inputs from peripheral organs, including the heart, kidneys, and adipose tissue, and humoral inputs from circulating factors such as proinflammatory cytokines and leptin, which are involved in the regulation of central sympathetic outflow. In the kidneys, changes in Wnt/β-catenin signaling have been reported in several hypertensive models. New findings on the renin-angiotensin-aldosterone system in the kidneys have also been reported. Sirtuin 6, which participates in various cellular functions, including DNA repair, has been shown to have protective effects on the vascular system. Skin water conservation, mediated by skin vasoconstriction and the accumulation of osmolytes such as sodium, has been found to contribute to hypertension. Studies of rivaroxaban and sodium-glucose cotransporter-2 inhibitors as drug repositioning candidates have been performed. Extracellular vesicles have been shown to be involved in novel diagnostic approaches and treatments for hypertension as well as other diseases. In gut microbiota studies, interactions between microbiota and antihypertensive drugs and potential pathophysiology linking microbiota and COVID-19 have been reported. It can be seen that inter-organ communication has received particular attention from these recent research topics. To truly understand the pathogenesis of hypertension and to develop treatments for conquering hypertension, interresearcher communication and collaboration should be further facilitated.

This mini-review focuses on recent topics on basic research in hypertension from the several points of view. The recent topics indicate that inter-organ communication has received particular attention. Interresearcher communication and collaboration should also be further facilitated to truly understand the complex pathogenesis of hypertension and to develop the treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hirooka Y. Sympathetic activation in hypertension: importance of the central nervous system. Am J Hypertens. 2020;33:914–26.
Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res. 2022;45:198–209.
Kario K, Hoshide S, Mogi M. A recent advance in Renal denervation to clinical practice. Hypertens Res. 2022 (e-pub ahead of print 20221005; https://doi.org/10.1038/s41440-022-01050-8).
Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ. Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf). 2015;213:778–94.
Zhu GQ, Xu Y, Zhou LM, Li YH, Fan LM, Wang W, et al. Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Exp Physiol. 2009;94:785–94.
Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–55.
Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–23.
Shibata R, Shinohara K, Ikeda S, Iyonaga T, Matsuura T, Kashihara S, et al. Transient receptor potential vanilloid 1-expressing cardiac afferent nerves may contribute to cardiac hypertrophy in accompany with an increased expression of brain-derived neurotrophic factor within nucleus tractus solitarius in a pressure overload model. Clin Exp Hypertens. 2022;44:249–57.
Xiong XQ, Chen WW, Zhu GQ. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol (Oxf). 2014;210:468–78.
Cao W, Shi M, Wu L, Li J, Yang Z, Liu Y, et al. Adipocytes initiate an adipose-cerebral-peripheral sympathetic reflex to induce insulin resistance during high-fat feeding. Clin Sci (Lond). 2019;133:1883–99.
Dalmasso C, Leachman JR, Osborn JL, Loria AS. Sensory signals mediating high blood pressure via sympathetic activation: role of adipose afferent reflex. Am J Physiol Regul Integr Comp Physiol. 2020;318:R379–r389.
Dalmasso C, Leachman JR, Ghuneim S, Ahmed N, Schneider ER, Thibault O, et al. Epididymal fat-derived sympathoexcitatory signals exacerbate neurogenic hypertension in obese male mice exposed to early life stress. Hypertension. 2021;78:1434–49.
Asirvatham-Jeyaraj N, Gauthier MM, Banek CT, Ramesh A, Garver H, Fink GD, et al. Renal denervation and celiac ganglionectomy decrease mean arterial pressure similarly in genetically hypertensive schlager (BPH/2J) mice. Hypertension. 2021;77:519–28.
Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension. 2013;61:806–11.
King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–56.
Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1965–73.
Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep. 2011;13:221–8.
Randhawa PK, Jaggi AS. TRPV1 channels in cardiovascular system: a double edged sword? Int J Cardiol. 2017;228:103–13.
Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–73.
Illig KA, Levy M, Sanchez L, Trachiotis GD, Shanley C, Irwin E, et al. An implantable carotid sinus stimulator for drug-resistant hypertension: surgical technique and short-term outcome from the multicenter phase II Rheos feasibility trial. J Vasc Surg. 2006;44:1213–8.
de Leeuw PW, Alnima T, Lovett E, Sica D, Bisognano J, Haller H, et al. Bilateral or unilateral stimulation for baroreflex activation therapy. Hypertension. 2015;65:187–92.
Domingos-Souza G, Santos-Almeida FM, Meschiari CA, Ferreira NS, Pereira CA, Pestana-Oliveira N, et al. The ability of baroreflex activation to improve blood pressure and resistance vessel function in spontaneously hypertensive rats is dependent on stimulation parameters. Hypertens Res. 2021;44:932–40.
Cao Y, Yu Y, Xue B, Wang Y, Chen X, Beltz TG, et al. IL (Interleukin)-17A acts in the brain to drive neuroinflammation, sympathetic activation, and hypertension. Hypertension. 2021;78:1450–62.
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25.
Wei SG, Yu Y, Felder RB. Blood-borne interleukin-1beta acts upon the subfornical organ to upregulate the sympathoexcitatory milieu of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2017. https://doi.org/10.1152/ajpregu.00211.2017.
Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–33.
Iyonaga T, Shinohara K, Mastuura T, Hirooka Y, Tsutsui H. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res. 2020;43:99–110.
Gruber T, Pan C, Contreras RE, Wiedemann T, Morgan DA, Skowronski AA, et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021;33:1155–.e1110.
Kasacka I, Piotrowska Z, Domian N, Acewicz M, Lewandowska A. Canonical Wnt signaling in the kidney in different hypertension models. Hypertens Res. 2021;44:1054–66.
Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17:172–84.
Nagasu H. Importance of wnt-catenin signaling in hypertensive kidney diseases. Hypertens Res. 2021;44:1546–7.
Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–9.
Matsuyama T, Ohashi N, Aoki T, Ishigaki S, Isobe S, Sato T, et al. Circadian rhythm of the intrarenal renin-angiotensin system is caused by glomerular filtration of liver-derived angiotensinogen depending on glomerular capillary pressure in adriamycin nephropathy rats. Hypertens Res. 2021;44:618–27.
Maeoka Y, Su XT, Wang WH, Duan XP, Sharma A, Li N, et al. Mineralocorticoid receptor antagonists cause natriuresis in the absence of aldosterone. Hypertension. 2022;79:1423–34.
Grootaert MOJ, Finigan A, Figg NL, Uryga AK, Bennett MR. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res. 2021;128:474–91.
Liu X, Jiang D, Huang W, Teng P, Zhang H, Wei C, et al. Sirtuin 6 attenuates angiotensin II-induced vascular adventitial aging in rat aortae by suppressing the NF-kappaB pathway. Hypertens Res. 2021;44:770–80.
Li W, Feng W, Su X, Luo D, Li Z, Zhou Y, et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease. J Clin Invest. 2022;132:e150051.
Ogura T, Kitada K, Morisawa N, Fujisawa Y, Kidoguchi S, Nakano D, et al. Contributions of renal water loss and skin water conservation to blood pressure elevation in spontaneously hypertensive rats. Hypertens Res. 2022; https://doi.org/10.1038/s41440-022-01044-6).
Kovarik JJ, Morisawa N, Wild J, Marton A, Takase-Minegishi K, Minegishi S, et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol (Oxf). 2021;232:e13629.
Wild J, Jung R, Knopp T, Efentakis P, Benaki D, Grill A, et al. Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol (Oxf). 2021;232:e13628.
Daci A, Da Dalt L, Alaj R, Shurdhiqi S, Neziri B, Ferizi R, et al. Rivaroxaban improves vascular response in LPS-induced acute inflammation in experimental models. PLoS One. 2020;15:e0240669.
Nakanishi N, Kaikita K, Ishii M, Oimatsu Y, Mitsuse T, Ito M, et al. Cardioprotective effects of rivaroxaban on cardiac remodeling after experimental myocardial infarction in mice. Circ Rep. 2020;2:158–66.
Narita M, Hanada K, Kawamura Y, Ichikawa H, Sakai S, Yokono Y, et al. Rivaroxaban attenuates cardiac hypertrophy by inhibiting protease-activated receptor-2 signaling in renin-overexpressing hypertensive mice. Hypertens Res. 2021;44:1261–73.
Kravtsova O, Bohovyk R, Levchenko V, Palygin O, Klemens CA, Rieg T, et al. SGLT2 inhibition effect on salt-induced hypertension, RAAS, and Na(+) transport in Dahl SS rats. Am J Physiol Ren Physiol. 2022;322:F692–f707.
Zhao Y, Li L, Lu Z, Hu Y, Zhang H, Sun F, et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin antagonizes salt-sensitive hypertension through modifying transient receptor potential channels 3 mediated vascular calcium handling. J Am Heart Assoc. 2022;11:e025328.
Ochiai-Homma F, Kuribayashi-Okuma E, Tsurutani Y, Ishizawa K, Fujii W, Odajima K, et al. Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism. Hypertens Res. 2021;44:1557–67.
Lugo-Gavidia LM, Burger D, Nolde JM, Carnagarin R, Chan J, Bosio E, et al. Platelet-derived extracellular vesicles correlate with therapy-induced nocturnal blood pressure changes. J Hypertens. 2022;40:2210–8.
Chi C, Fu H, Li YH, Zhang GY, Zeng FY, Ji QX, et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur Heart J. 2022; https://doi.org/10.1093/eurheartj/ehac431).
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17.
Wang C, Wu H, Xing Y, Ye Y, He F, Yin Q, et al. Endothelial-derived extracellular microRNA-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells. Sci Rep. 2022;12:344.
Mishima E, Abe T. Role of the microbiota in hypertension and antihypertensive drug metabolism. Hypertens Res. 2022;45:246–53.
Kyoung J, Atluri RR, Yang T. Resistance to antihypertensive drugs: is gut microbiota the missing link? Hypertension. 2022;79:2138–47.
Wu H, Lam TYC, Shum TF, Tsai TY, Chiou J. Hypotensive effect of captopril on deoxycorticosterone acetate-salt-induced hypertensive rat is associated with gut microbiota alteration. Hypertens Res. 2022;45:270–82.
Yang T, Mei X, Tackie-Yarboi E, Akere MT, Kyoung J, Mell B, et al. Identification of a gut commensal that compromises the blood pressure-lowering effect of Ester angiotensin-converting enzyme inhibitors. Hypertension . 2022;79:1591–601.
Kai H, Kai M, Niiyama H, Okina N, Sasaki M, Maeda T, et al. Overexpression of angiotensin-converting enzyme 2 by renin-angiotensin system inhibitors. Truth or myth? A systematic review of animal studies. Hypertens Res. 2021;44:955–68.
Shibata S, Arima H, Asayama K, Hoshide S, Ichihara A, Ishimitsu T, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertension Res. 2020;43:1028–46.
Ishida M. How to deal with hypertension in the COVID-19 era—the impact “ON” and “OF” hypertension. Hypertension Res. 2022;45:548–50.
Wojciechowska W, Terlecki M, Klocek M, Pac A, Olszanecka A, Stolarz-Skrzypek K, et al. Impact of arterial hypertension and use of antihypertensive pharmacotherapy on mortality in patients hospitalized due to COVID-19: the CRACoV-HHS study. Hypertension. 2022;79:2601–10.
Li J, Stevens BR, Richards EM, Raizada MK. SARS-CoV-2 receptor ACE2 (Angiotensin-Converting Enzyme 2) is upregulated in colonic organoids from hypertensive rats. Hypertension. 2020;76:e26–8.
Sharma RK, Stevens BR, Obukhov AG, Grant MB, Oudit GY, Li Q, et al. ACE2 (Angiotensin-Converting Enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension. 2020;76:651–61.
Li J, Richards EM, Handberg EM, Pepine CJ, Raizada MK. Butyrate regulates COVID-19-relevant genes in gut epithelial organoids from normotensive rats. Hypertension. 2021;77:e13–6.
Acknowledgements
I sincerely appreciate the editors of Hypertension Research for giving me the opportunity to write this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shinohara, K. Emerging topics on basic research in hypertension: interorgan communication and the need for interresearcher collaboration. Hypertens Res 46, 638–645 (2023). https://doi.org/10.1038/s41440-023-01176-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-023-01176-3