Abstract
Pregnancy-induced hypertension (PIH), a prominent determinant of maternal mortality and morbidity worldwide, is hindered by the absence of efficacious biomarkers for early diagnosis, contributing to suboptimal outcomes. Here, we explored potential causal relationships between blood metabolites and the risk of PIH using Mendelian randomization (MR). We employed a two-sample univariable MR approach to empirically estimate the causal relationships between 249 circulating metabolites and PIH. Inverse variance weighted, MR-egger, weight median, simple mode, and weighted mode methods were used for causal estimates. The exposure-to-outcome directionality was confirmed with the MR Steiger test. The Bayesian model averaging MR (MR-BMA) method was applied to detect the predominant causal metabolic traits with alignment for pleiotropy effects. In the primary analysis, analyzing 249 metabolites, we identified 25 causally linked to PIH, including 11 lipid-related traits and 6 associated with fatty acid (un)saturation. Importantly, MR-BMA analyses corroborated the total concentration of branched-chain amino acids(total-BCAA) to be the highest rank causal metabolite, followed by leucine (Leu), phospholipids to total lipids ratio in medium LDL (M-LDL-PL-pct), and Val (all P < 0.05). The directionality of causality predicted by univariable MR and MR-BMA for these metabolites remained consistent. This study highlights the causal connection between metabolites and PIH risk. It highlighted BCAAs as the strongest causal candidates warranting further investigation. Since PIH typically occurs in the second and third trimesters, extending these findings could inform earlier strategies to reduce its risk.

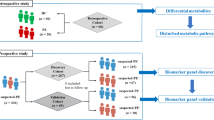
Directed acyclic graph of the MR framework investigating the causal relationship between metabolites and PIH. MR: Mendelian randomization; GIVs: genetic instrument variables; SNPs: single-nucleotide polymorphism; IVW: inverse variance weighted; WM: weighted median; PIH: pregnancy-induced hypertension; SM: significant metabolite; MR-BMA: Bayesian model averaging MR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ou M, Zhao H, Ji G, Zhao X, Zhang Q. Long noncoding RNA MALAT1 contributes to pregnancy-induced hypertension development by enhancing oxidative stress and inflammation through the regulation of the miR-150-5p/ET-1 axis. FASEB J. 2020;34:6070–85.
Wang R, Chen L, Wang X, Liu Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc Res. 2021;135:104130.
Wei W, Wang X, Zhou Y, Shang X, Yu H. The genetic risk factors for pregnancy-induced hypertension: evidence from genetic polymorphisms. FASEB J. 2022;36:e22413.
Zhao A, Qi Y, Liu K. CLDN3 expression and function in pregnancy-induced hypertension. Exp Ther Med. 2020;20:3798–806.
Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-Induced hypertension. Hormones. 2015;14:211–23.
Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:l5119.
Qu H, Khalil RA. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am J Physiol Heart Circ Physiol. 2020;319:H661–h81.
Chen A, Zhao H, Wang J, Zhang R, Liu J, Zhao X, et al. Haplotype analysis of candidate genes involved in inflammation and oxidative stress and the susceptibility to preeclampsia. J Immunol Res. 2020;2020:4683798.
Yang J, Shang J, Zhang S, Li H, Liu H. The role of the renin-angiotensin-aldosterone system in preeclampsia: genetic polymorphisms and microRNA. J Mol Endocrinol. 2013;50:R53–66.
Liu Q, Wang XX, Zhang YK, Li JH, Wang L.Correlation between pregnancy-induced hypertension and age in pregnant women from Hebei province.Zhonghua Liu Xing Bing Xue Za Zhi. 2016;39:1270–3.
Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. 2014;9:e100180.
Suhre K, Raffler J, Kastenmüller G. Biochemical insights from population studies with genetics and metabolomics. Arch Biochem Biophys. 2016;589:168–76.
Wang Q, Würtz P, Auro K, Mäkinen VP, Kangas AJ, Soininen P, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC Med. 2016;14:205.
White SL, Pasupathy D, Sattar N, Nelson SM, Lawlor DA, Briley AL, et al. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia. 2017;60:1903–12.
Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021;71:333–58.
Agrawal S, Cerdeira AS, Redman C, Vatish M. Meta-analysis and systematic review to assess the role of soluble FMs-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension. 2018;71:306–16.
Sovio U, McBride N, Wood AM, Masconi KL, Cook E, Gaccioli F, et al. 4-Hydroxyglutamate is a novel predictor of pre-eclampsia. Int J Epidemiol. 2020;49:301–11.
Kuc S, Koster MP, Pennings JL, Hankemeier T, Berger R, Harms AC, et al. Metabolomics profiling for identification of novel potential markers in early prediction of preeclampsia. PLoS One. 2014;9:e98540.
Khalil AA, Tsikas D, Akolekar R, Jordan J, Nicolaides KH. Asymmetric dimethylarginine, arginine and homoarginine at 11-13 weeks’ gestation and preeclampsia: a case-control study. J Hum Hypertens. 2013;27:38–43.
Wojcik-Baszko D, Charkiewicz K, Laudanski P. Role of dyslipidemia in preeclampsia—a review of lipidomic analysis of blood, placenta, syncytiotrophoblast microvesicles and umbilical cord artery from women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018;139:19–23.
Dobierzewska A, Soman S, Illanes SE, Morris AJ. Plasma cross-gestational sphingolipidomic analyses reveal potential first-trimester biomarkers of preeclampsia. PLoS One. 2017;12:e0175118.
Bahado-Singh RO, Akolekar R, Mandal R, Dong E, Xia J, Kruger M, et al. First-trimester metabolomic detection of late-onset preeclampsia. Am J Obstet Gynecol. 2013;208:58.e1–7.
Bahado-Singh RO, Syngelaki A, Mandal R, Graham SF, Akolekar R, Han B, et al. Metabolomic determination of pathogenesis of late-onset preeclampsia. J Matern Fetal Neonatal Med. 2017;30:658–64.
Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–52.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–6.
Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. Jama. 2008;299:2524–32.
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–80.
Zuber V, Colijn JM, Klaver C, Burgess S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat Commun. 2020;11:29.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenomenon. Elife. 2018;7:e34408.
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186.
Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35:1880–906.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89.
Sivanand S, Vander Heiden MG. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell. 2020;37:147–56.
Evans RW, Powers RW, Ness RB, Cropcho LJ, Daftary AR, Harger GF, et al. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction. 2003;125:785–90.
Lima VJ, Andrade CR, Ruschi GE, Sass N. Serum lipid levels in pregnancies complicated by preeclampsia. Sao Paulo Med J. 2011;129:73–6.
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211.
Austdal M, Skråstad RB, Gundersen AS, Austgulen R, Iversen AC, Bathen TF. Metabolomic biomarkers in serum and urine in women with preeclampsia. PLoS One. 2014;9:e91923.
Yadav K, Aggarwal S, Verma K. Serum βhCG and lipid profile in early second trimester as predictors of pregnancy-induced hypertension. J Obstet Gynaecol India. 2014;64:169–74.
Murmu S, Dwivedi J. Second-trimester maternal serum beta-human chorionic gonadotropin and lipid profile as a predictor of gestational hypertension, preeclampsia, and eclampsia: a prospective observational study. Int J Appl Basic Med Res. 2020;10:49–53.
Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17:574–81.
Kemse NG, Kale AA, Joshi SR. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension Wistar rats improves IL10 and VEGF levels. Prostaglandins Leukot Essent Fatty Acids. 2016;104:25–32.
Kemse NG, Kale AA, Joshi SR. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy-induced hypertension. PLoS One. 2014;9:e111902.
Wadhwani NS, Narang AS, Mehendale SS, Wagh GN, Gupte SA, Joshi SR. Reduced maternal erythrocyte long-chain polyunsaturated fatty acids exist in early pregnancy in preeclampsia. Lipids. 2016;51:85–94.
Mackay VA, Huda SS, Stewart FM, Tham K, McKenna LA, Martin I, et al. Preeclampsia is associated with compromised maternal synthesis of long-chain polyunsaturated fatty acids, leading to offspring deficiency. Hypertension. 2012;60:1078–85.
Wadhwani N, Patil V, Pisal H, Joshi A, Mehendale S, Gupte S, et al. Altered maternal proportions of long chain polyunsaturated fatty acids and their transport leads to disturbed fetal stores in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2014;91:21–30.
Wang Y, Walsh SW, Kay HH. Placental tissue levels of nonesterified polyunsaturated fatty acids in normal and preeclamptic pregnancies. Hypertens Preg. 2005;24:235–45.
Williams MA, Zingheim RW, King IB, Zebelman AM. Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology. 1995;6:232–7.
Al MD, van Houwelingen AC, Badart-Smook A, Hasaart TH, Roumen FJ, Hornstra G. The essential fatty acid status of mother and child in pregnancy-induced hypertension: a prospective longitudinal study. Am J Obstet Gynecol. 1995;172:1605–14.
Bakouei F, Delavar MA, Mashayekh-Amiri S, Esmailzadeh S, Taheri Z. Efficacy of n-3 fatty acids supplementation on the prevention of pregnancy induced-hypertension or preeclampsia: a systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2020;59:8–15.
Chen B, Ji X, Zhang L, Hou Z, Li C, Tong Y. Fish oil supplementation does not reduce risks of gestational diabetes mellitus, pregnancy-induced hypertension, or pre-eclampsia: a meta-analysis of randomized controlled trials. Med Sci Monit. 2015;21:2322–30.
Hao Y, Sun X, Wen N, Song D, Li H. Effects of n-3 polyunsaturated fatty acid supplementation on pregnancy outcomes: a systematic review and meta-analysis. Arch Med Sci. 2022;18:890–9.
Zhou SJ, Yelland L, McPhee AJ, Quinlivan J, Gibson RA, Makrides M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am J Clin Nutr. 2012;95:1378–84.
Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. 2019;81:139–64.
Tom A, Nair KS. Assessment of branched-chain amino acid status and potential for biomarkers. J Nutr. 2006;136:324s–30s.
Funding
This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2023QH345).
Author information
Authors and Affiliations
Contributions
JG and XFZ: Study conception, design, Analyses and Draft; XD: Analyses; WSL and LKL: Supervision and validation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The datasets utilized in this study were obtained from the IEU Open GWAS database (https://gwas.mrcieu.ac.uk/). These public databases permit researchers to access and analyze public datasets for scientific research, rendering ethics approval unnecessary.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Zheng, X., Du, X. et al. BMA-based Mendelian randomization identifies blood metabolites as causal candidates in pregnancy-induced hypertension. Hypertens Res 47, 2549–2560 (2024). https://doi.org/10.1038/s41440-024-01787-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01787-4