Abstract
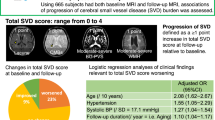
We investigated the effects of individual and cumulative cerebral small vessel disease (SVD) markers on long-term clinical outcomes in spontaneous intracerebral hemorrhage (sICH) patients. This prospective, single-center cohort study was conducted from 2012 to 2019. SVD markers, including lacunae, cerebral microbleeds, white matter hyperintensity (WMH), and perivascular spaces in the basal ganglia, were assessed to calculate a summary SVD score. Patients were categorized into severe (score ≥3) and non-severe (score 0–2) SVD burden groups. Functional prognosis was defined as recovery, no change, or decline based on modified Rankin Scale changes at 2 years after discharge, excluding death. Associations of SVD burden and individual SVD markers with outcomes were evaluated using Cox proportional hazards modeling for recurrent stroke and all-cause mortality, and using ordinal logistic regression for functional prognosis. Among 155 sICH patients who underwent MRI, 98 showed severe SVD burden. Recurrent stroke and all-cause mortality rates were 2.2 and 8.3 per 100 patient-years, respectively, over a median 2.1-year follow-up. In terms of functional prognosis, 57 patients (51.8%) recovered, 32 (29.1%) showed no change, and 21 (19.1%) declined. A significant association was apparent between severe SVD burden and poorer functional prognosis (odds ratio [OR] 2.48, 95% confidence interval [CI] 1.04–6.04; p = 0.042), particularly with moderate-to-severe WMH (OR 2.54, 95%CI 1.02–6.54; p = 0.048). The cumulative effects of SVD markers inhibited long-term functional recovery in sICH patients. Severe SVD burden, as well as moderate-to-severe WMH, can be indicators of long-term prognosis after sICH.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Analyses for the HAGAKURE study are on-going; however, once completed, the data generated from this work will be available upon reasonable request.
References
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Yakushiji Y, Werring DJ. Cerebrovascular disease: lobar cerebral microbleeds signal early cognitive impairment. Nat Rev Neurol. 2016;12:680–2.
Suzuyama K, Yakushiji Y, Ogata A, Nishihara M, Eriguchi M, Kawaguchi A, et al. Total small vessel disease score and cerebro-cardiovascular events in healthy adults: the Kashima scan study. Int J Stroke. 2020;15:973–9.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83:1228–34.
Ikeda S, Yakushiji Y, Tanaka J, Nishihara M, Ogata A, Eriguchi M, et al. Hypertension, cerebral Amyloid, aGe Associated Known neuroimaging markers of cerebral small vessel disease Undertaken with stroke REgistry (HAGAKURE) prospective cohort study: baseline characteristics and association of cerebral small vessel disease with prognosis in an ischemic stroke cohort. Front Aging Neurosci. 2023;15:1117851.
Charidimou A, Schmitt A, Wilson D, Yakushiji Y, Gregoire SM, Fox Z, et al. The Cerebral Haemorrhage Anatomical RaTing inStrument (CHARTS): development and assessment of reliability. J Neurol Sci 2017;372:178–83.
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66.
Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–50.
Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology. 2017;88:2260–7.
Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis. 2010;29:137–9.
Lioutas VA, Wu B, Norton C, Helenius J, Modak J, Selim M. Cerebral small vessel disease burden and functional and radiographic outcomes in intracerebral hemorrhage. J Neurol. 2018;265:2803–14.
Cheng Z, Zhang W, Zhan Z, Xia L, Han Z. Cerebral small vessel disease and prognosis in intracerebral haemorrhage: a systematic review and meta-analysis of cohort studies. Eur J Neurol. 2022;29:2511–25.
Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32.
Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–99.
Borschmann KN, Hayward KS. Recovery of upper limb function is greatest early after stroke but does continue to improve during the chronic phase: a two-year, observational study. Physiotherapy. 2020;107:216–23.
Mutai H, Furukawa T, Nakanishi K, Hanihara T. Longitudinal functional changes, depression, and health-related quality of life among stroke survivors living at home after inpatient rehabilitation. Psychogeriatrics. 2016;16:185–90.
Löfgren B, Nyberg L, Mattsson M, Gustafson Y. Three years after in-patient stroke rehabilitation: a follow-up study. Cerebrovasc Dis. 1999;9:163–70.
Meyer S, Verheyden G, Brinkmann N, Dejaeger E, De Weerdt W, Feys H, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke. 2015;46:1613–9.
van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37:167–71.
Pettersen R, Dahl T, Wyller TB. Prediction of long-term functional outcome after stroke rehabilitation. Clin Rehabil. 2002;16:149–59.
Rodrigues MA, Samarasekera NE, Lerpiniere C, Perry LA, Moullaali TJ, Loan JJM, et al. Association between computed tomographic biomarkers of cerebral small vessel diseases and long-term outcome after spontaneous intracerebral hemorrhage. Ann Neurol. 2021;89:266–79.
Uniken Venema SM, Marini S, Lena UK, Morotti A, Jessel M, Moomaw CJ, et al. Impact of cerebral small vessel disease on functional recovery after intracerebral hemorrhage. Stroke. 2019;50:2722–8.
Shah VA, Thompson RE, Yenokyan G, Acosta JN, Avadhani R, Dlugash R, et al. One-year outcome trajectories and factors associated with functional recovery among survivors of intracerebral and intraventricular hemorrhage with initial severe disability. JAMA Neurol. 2022;79:856–68.
de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83.
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Valenti R, Reijmer YD, Charidimou A, Boulouis G, Martinez SR, Xiong L, et al. Total small vessel disease burden and brain network efficiency in cerebral amyloid angiopathy. J Neurol Sci 2017;382:10–2.
van Meer MP, Otte WM, van der Marel K, Nijboer CH, Kavelaars A, van der Sprenkel JW, et al. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J Neurosci. 2012;32:4495–507.
Förster A, Griebe M, Ottomeyer C, Rossmanith C, Gass A, Kern R, et al. Cerebral network disruption as a possible mechanism for impaired recovery after acute pontine stroke. Cerebrovasc Dis. 2011;31:499–505.
Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012;26:7–19.
Molad J, Kliper E, Korczyn AD, Ben Assayag E, Ben Bashat D, Shenhar-Tsarfaty S, et al. Only white matter hyperintensities predicts post-stroke cognitive performances among cerebral small vessel disease markers: results from the TABASCO study. J Alzheimers Dis. 2017;56:1293–9.
Pasi M, Sugita L, Xiong L, Charidimou A, Boulouis G, Pongpitakmetha T, et al. Association of cerebral small vessel disease and cognitive decline after intracerebral hemorrhage. Neurology. 2021;96:e182–e92.
Pasi M, Casolla B, Kyheng M, Boulouis G, Kuchcinski G, Moulin S, et al. Long-term functional decline of spontaneous intracerebral haemorrhage survivors. J Neurol Neurosurg Psychiatry. 2021;92:249–54.
Toyoda K, Yoshimura S, Nakai M, Koga M, Sasahara Y, Sonoda K, et al. Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol. 2022;79:61–9.
Sreekrishnan A, Dearborn JL, Greer DM, Shi FD, Hwang DY, Leasure AC, et al. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care. 2016;25:384–91.
Nandigam RNK, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am J Neuroradiol. 2009;30:338–43.
Yakushiji Y, Tanaka J, Wilson D, Charidimou A, Noguchi T, Kawashima M, et al. Proportion of intracerebral haemorrhage due to cerebral amyloid angiopathy in the East and West: comparison between single hospital centres in Japan and the United Kingdom. J Neurol Sci. 2020;416:117037.
Yakushiji Y, Wilson D, Ambler G, Charidimou A, Beiser A, van Buchem MA, et al. Distribution of cerebral microbleeds in the East and West: individual participant meta-analysis. Neurology. 2019;92:e1086–e97.
Acknowledgements
We are indebted to Dr. Kiku Uwatoko, Dr. Hirome Minagawa, Kazuhiro Kawamoto, Yoko Wakabayashi, and Miki Fujii for data management.
Funding
The HAGAKURE was organized by a central coordinating center located at Saga University Hospital and an associated center at Kansai Medical University, with funding support by a Grant-in-Aid for Scientific Research (C), JSPS KAKENHI (grant nos. 15K10364 and 21K10510).
Author information
Authors and Affiliations
Contributions
Study conception: SI, YY, JT, and HH. Data acquisition: SI, YY, JT, ME, SO, MK, KS, MM, CS, TI, YN, NO, MY, and YK. Analysis and interpretation of data: SI, YY, JT, MN, and AO. Drafting the manuscript: SI, YY, and HH. Supervision of the study: YY, HI, TA, HK, and HH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeda, S., Yakushiji, Y., Tanaka, J. et al. Cerebral small vessel disease markers and long-term prognosis in spontaneous intracerebral hemorrhage: the HAGAKURE-ICH study. Hypertens Res 48, 233–243 (2025). https://doi.org/10.1038/s41440-024-01906-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01906-1
Keywords
This article is cited by
-
Brain and hypertension: from sympathetic outflow to brain-focused blood pressure management
Hypertension Research (2026)
-
Assessing etiological classification systems and their relationship with neurological deterioration in patients with intracerebral hemorrhage
Scientific Reports (2025)
-
SERPINA3 predicts long-term neurological outcomes and mortality in patients with intracerebral hemorrhage
Cell Death & Disease (2025)
-
Exploring the impact of cerebral small vessel disease on long-term prognosis in spontaneous intracerebral hemorrhage
Hypertension Research (2025)