Abstract
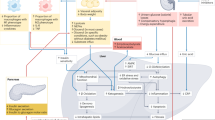
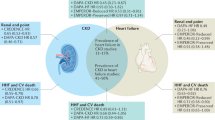
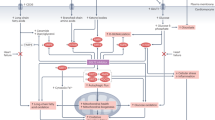
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to suppress cardiovascular events and are widely used for treating diabetes, chronic heart failure and chronic kidney disease. Although the underlying mechanisms by which SGLT2 inhibitors suppress cardiovascular events are not entirely clear, several mechanisms have been proposed to explain the cardiorenal protective effects of SGLT2 inhibitors. One of these involves sympathoinhibition. In vitro, SGLT2 expression is upregulated by norepinephrine, and SGLT2 inhibitors have been shown to attenuate SGLT2 expression and normalize the diuretic response to volume expansion with isotonic saline in rats with heart failure. These findings suggest that inhibition of renal sympathetic nerve activity is the mechanism underlying the beneficial effects of SGLT2 inhibitors on heart failure. Increased resting afferent renal nerve activity has been observed in several disease models, including models of hypertension, heart failure, and kidney disease, and might induce augmented sympathetic outflow via the central nervous system. SGLT2 inhibitors may suppress afferent renal nerve activity via intrarenal environmental modifications such as renal tissue hypoxia, inflammation, oxidative stress, mitochondrial function, and congestion, thereby inhibiting sympathetic outflow to the peripheral organs, including the heart and kidneys. On the other hand, SGLT2 is also expressed in the brain, and electrophysiological techniques in rats have shown that SGLT2 inhibitors suppress the activities of the rostral ventrolateral medulla neurons which project to the sympathetic preganglionic nuclei of the spinal cord to control sympathetic outflow, suggesting decreased sympathetic nerve activities. This mini review focuses on the bidirectional interaction between SGLT2 and the sympathetic nervous system and introduces recent related findings from Hypertension Research and other journals.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14.
Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J. 2016;37:3192–200.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–57.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413–24.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451–61.
Esler M. The 2009 Carl Ludwig Lecture: pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010;108:227–37.
Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–66.
Grassi G, Quarti-Trevano F, Esler MD. Sympathetic activation in congestive heart failure: an updated overview. Heart Fail Rev. 2019. https://doi.org/10.1007/s10741-019-09901-2.
Rafiq K, Fujisawa Y, Sherajee SJ, Rahman A, Sufiun A, Kobori H, et al. Role of the renal sympathetic nerve in renal glucose metabolism during the development of type 2 diabetes in rats. Diabetologia. 2015;58:2885–98.
Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059–68.
Katsurada K, Nandi SS, Sharma NM, Patel KP. Enhanced Expression and Function of Renal SGLT2 (Sodium-Glucose Cotransporter 2) in Heart Failure: Role of Renal Nerves. Circ Heart Fail. 2021;14:e008365.
Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Ren Physiol. 2014;306:F194–204.
Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Ren Physiol. 2014;307:F317–25.
Ji W, Zhao M, Wang M, Yan W, Liu Y, Ren S, et al. Effects of canagliflozin on weight loss in high-fat diet-induced obese mice. PLoS One. 2017;12:e0179960.
Rahman A, Kittikulsuth W, Fujisawa Y, Sufiun A, Rafiq K, Hitomi H, et al. Effects of diuretics on sodium-dependent glucose cotransporter 2 inhibitor-induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J Hypertens. 2016;34:893–906.
Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Ren Physiol. 2013;304:F156–67.
Ma C, de Baaij JHF, Millar PJ, Gault VA, de Galan BE, Bindels RJM, et al. Effect of Dapagliflozin Treatment on the Expression of Renal Sodium Transporters/Channels on High-Fat Diet Diabetic Mice. Nephron. 2019;142:51–60.
Albertoni Borghese MF, Majowicz MP, Ortiz MC, Passalacqua MdelR, Sterin Speziale NB, Vidal NA. Expression and activity of SGLT2 in diabetes induced by streptozotocin: relationship with the lipid environment. Nephron Physiol. 2009;112:p45–52.
Borges-Junior FA, Silva Dos Santos D, Benetti A, Polidoro JZ, Wisnivesky ACT, Crajoinas RO, et al. Empagliflozin Inhibits Proximal Tubule NHE3 Activity, Preserves GFR, and Restores Euvolemia in Nondiabetic Rats with Induced Heart Failure. J Am Soc Nephrol. 2021;32:1616–29.
Endo A, Hirose T, Sato S, Ito H, Takahashi C, Ishikawa R, et al. Sodium glucose cotransporter 2 inhibitor suppresses renal injury in rats with renal congestion. Hypertens Res. 2024;47:33–45.
Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl Sci. 2020;5:169–79.
Patel KP. Central alpha-2 adrenergic mechanisms in the renal nerve mediated natriuresis and diuresis produced by acute volume expansion. J Auton Nerv Syst. 1991;36:47–54.
Zeigler DW, Patel KP. Reduced renal responses to an acute saline load in obese Zucker rats. Am J Physiol. 1991;261:R712–8.
Badoer E, Moguilevski V, McGrath BP. Cardiac afferents play the dominant role in renal nerve inhibition elicited by volume expansion in the rabbit. Am J Physiol. 1998;274:R383–8.
Widdop RE, Verberne AJ, Jarrott B, Louis WJ. Impaired arterial baroreceptor reflex and cardiopulmonary vagal reflex in conscious spontaneously hypertensive rats. J Hypertens. 1990;8:269–75.
Huang BS, Leenen FH. Brain ‘ouabain,’ sodium, and arterial baroreflex in spontaneously hypertensive rats. Hypertension. 1995;25:814–7.
DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol. 1994;266:R27–39.
Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Ren Physiol. 2006;291:F1148–56.
Kim HK, Ishizawa R, Fukazawa A, Wang Z, Bezan Petric U, Hu MC, et al. Dapagliflozin Attenuates Sympathetic and Pressor Responses to Stress in Young Prehypertensive Spontaneously Hypertensive Rats. Hypertension. 2022;79:1824–34.
Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108:8030–5.
Colina C, Puhl HL 3rd, Ikeda SR. Selective tracking of FFAR3-expressing neurons supports receptor coupling to N-type calcium channels in mouse sympathetic neurons. Sci Rep. 2018;8:17379.
Tardif A, Julien N, Pelletier A, Thibault G, Srivastava AK, Chiasson JL, et al. Chronic exposure to beta-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2001;281:E1205–12.
Velazquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: involvement of multiple isoforms. Pharm Res. 2007;55:578–89.
Yoshikawa T, Kishi T, Shinohara K, Takesue K, Shibata R, Sonoda N, et al. Arterial pressure lability is improved by sodium-glucose cotransporter 2 inhibitor in streptozotocin-induced diabetic rats. Hypertens Res. 2017;40:646–51.
Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–6.
Verma S. Are the Cardiorenal Benefits of SGLT2 Inhibitors Due to Inhibition of the Sympathetic Nervous System? JACC Basic Transl Sci. 2020;5:180–2.
Spallone V, Valensi P. SGLT2 inhibitors and the autonomic nervous system in diabetes: A promising challenge to better understand multiple target improvement. Diabetes Metab. 2021;47:101224.
Katsurada K, Ogoyama Y, Imai Y, Patel KP, Kario K. Renal denervation based on experimental rationale. Hypertens Res. 2021;44:1385–94.
Patel KP, Katsurada K, Zheng H. Cardiorenal Syndrome: The Role of Neural Connections Between the Heart and the Kidneys. Circ Res. 2022;130:1601–17.
Zheng H, Katsurada K, Liu X, Knuepfer MM, Patel KP. Specific Afferent Renal Denervation Prevents Reduction in Neuronal Nitric Oxide Synthase Within the Paraventricular Nucleus in Rats With Chronic Heart Failure. Hypertension. 2018;72:667–75.
Katsurada K, Nandi SS, Zheng H, Liu X, Sharma NM, Patel KP. GLP-1 mediated diuresis and natriuresis are blunted in heart failure and restored by selective afferent renal denervation. Cardiovasc Diabetol. 2020;19:57.
Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, et al. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68:1415–23.
Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol. 2019;122:358–67.
Gauthier MM, Dennis MR, Morales MN, Brooks HL, Banek CT. Contributions of afferent and sympathetic renal nerves to cystogenesis and arterial pressure regulation in a preclinical model of autosomal recessive polycystic kidney disease. Am J Physiol Ren Physiol. 2022;322:F680–F91.
Tyshynsky R, Sensarma S, Riedl M, Bukowy J, Schramm LP, Vulchanova L, et al. Periglomerular afferent innervation of the mouse renal cortex. Front Neurosci. 2023;17:974197.
Evans LC, Dayton A, Osborn JW. Renal nerves in physiology, pathophysiology and interoception. Nat Rev Nephrol. 2024. https://doi.org/10.1038/s41581-024-00893-3.
Packer M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation. 2022;146:1383–405.
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Hirooka Y. Sympathetic Activation in Hypertension: Importance of the Central Nervous System. Am J Hypertens. 2020;33:914–26.
Chiba Y, Sugiyama Y, Nishi N, Nonaka W, Murakami R, Ueno M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brains. Neuropathology. 2020;40:482–91.
Nguyen T, Wen S, Gong M, Yuan X, Xu D, Wang C, et al. Dapagliflozin Activates Neurons in the Central Nervous System and Regulates Cardiovascular Activity by Inhibiting SGLT-2 in Mice. Diabetes Metab Syndr Obes. 2020;13:2781–99.
Oshima N, Onimaru H, Yamashiro A, Goto H, Tanoue K, Fukunaga T, et al. SGLT2 and SGLT1 inhibitors suppress the activities of the RVLM neurons in newborn Wistar rats. Hypertens Res. 2024;47:46–54.
Al Hamed FA, Elewa H. Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke. Clin Ther. 2020;42:e242–e9.
Pawlos A, Broncel M, Wozniak E, Gorzelak-Pabis P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules. 2021;26:7213.
Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148.
Hamaoka T, Murai H, Hirai T, Sugimoto H, Mukai Y, Inoue O, et al. Different Responses of Muscle Sympathetic Nerve Activity to Dapagliflozin Between Patients With Type 2 Diabetes With and Without Heart Failure. J Am Heart Assoc. 2021;10:e022637.
Takeshita Y, Nomura C, Murai H, Mukai Y, Hirai T, Hamaoka T, et al. Study Protocol for the Pleiotropic Effects of Sodium-Glucose Cotransporter 2 Inhibitor on Organ-Specific Sympathetic Nerve Activity and Insulin Sensitivity in Participants with Type 2 Diabetes. Diabetes Ther. 2024;15:269–80.
Funding
This work was supported in part by JSPS KAKENHI Grant Number JP24K11196, Japan Diabetes Foundation, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, and Japan Research Foundation for Clinical Pharmacology (to KK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katsurada, K. Interaction between SGLT2 and the sympathetic nervous system in normal and various cardiovascular metabolic disease states. Hypertens Res 48, 2072–2078 (2025). https://doi.org/10.1038/s41440-025-02216-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02216-w
Keywords
This article is cited by
-
Brain and hypertension: from sympathetic outflow to brain-focused blood pressure management
Hypertension Research (2026)