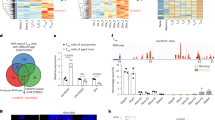
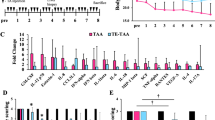
Abstract
Tumor necrosis factor α (TNFα) regulates inflammation in metabolic diseases and probably aging-associated inflammation. Here, TNFα´s role in aging-related liver inflammation and fibrosis and underlying mechanisms was assessed in mice. In male C57BL/6J mice, aging increased hepatic inflammation, senescence markers p16 and p21 and Tnfa mRNA expression in liver tissue. In a second study, 4 and 24-month-old TNFα-/- and wild-type (WT) mice were compared for senescence, liver damage, intestinal barrier function, and microbiota composition. 24-month-old TNFα-/- mice were significantly protected from the aging-associated increase in hepatic senescence, inflammation and fibrosis found in WT mice. This protection was related with preserved stem cell marker expression, maintained small intestinal barrier function and lower bacterial endotoxin in portal blood. While differing from young mice, intestinal microbiota composition of old TNFα-/- mice differed markedly from age-matched WT mice. Also, TNFα was found to alter permeability and tight junction protein levels being reversed by the presence of an JNK inhibitor in an ex vivo intestinal tissue model. Taken together, our results suggest that TNFα plays a key role in the development of aging-related liver decline in male mice.

Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/ Supplementary Material and raw sequences were deposited to the European Nucleotide Archive (ENA) under accession number PRJEB76497. Further inquiries can be directed to the corresponding author.
References
World Health Organization. Ageing. WHO, 2021. https://www.who.int/health-topics/ageing (accessed 25 January 2023).
GBD Diseases and Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222 (2020).
He, Q. J. et al. Recent advances in age-related metabolic dysfunction-associated steatotic liver disease. World J. Gastroenterol. 30, 652–662 (2024).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Yan, Z., Cai, M., Han, X., Chen, Q. & Lu, H. The Interaction between age and risk factors for diabetes and prediabetes: a community-based cross-sectional study. Diab. Metab. Syndr. Obes. 16, 85–93 (2023).
Rinella, M. E. et al. A multisociety delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9 (2014).
Baumann, A. et al. Microbiota profiling in aging-associated inflammation and liver degeneration. Int. J. Med. Microbiol. IJMM 311, 151500 (2021).
Brandt, A. et al. Impairments of intestinal arginine and no metabolisms trigger aging-associated intestinal barrier dysfunction and ‘inflammaging. Redox Biol. 58, 102528 (2022).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Tiegs, G. & Horst, A. K. Tnf in the liver: targeting a central player in inflammation. Semin. Immunopathol. 44, 445–459 (2022).
van Loo, G. & Bertrand, M. J. M. Death by Tnf: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Jin, C. J. et al. Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G736–g747 (2020).
Bauernfeind, F., Niepmann, S., Knolle, P. A. & Hornung, V. Aging-associated TNF production primes inflammasome activation and nlrp3-related metabolic disturbances. J. Immun. 197, 2900–2908 (2016).
Alvarez-Rodríguez, L., López-Hoyos, M., Muñoz-Cacho, P. & Martínez-Taboada, V. M. Aging is associated with circulating cytokine dysregulation. Cell. Immunol. 273, 124–132 (2012).
Burger, K. et al. Tnfα is a key trigger of inflammation in diet-induced non-obese masld in mice. Redox Biol. 66, 102870 (2023).
Barbuio, R., Milanski, M., Bertolo, M. B., Saad, M. J. & Velloso, L. A. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J. Endocrinol. 194, 539–550 (2007).
Li, Z. et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37, 343–350 (2003).
Cure, E. et al. Protective effect of infliximab on methotrexate-induced liver injury in rats: unexpected drug interaction. J. Cancer Res. Ther. 11, 164–169 (2015).
Noth, R. et al. Anti-Tnf-Α antibodies improve intestinal barrier function in Crohn’s disease. J. Crohn’s. Colitis 6, 464–469 (2012).
Kurioka, A. & Klenerman, P. Aging unconventionally: Γδ T cells, inkt cells, and mait cells in aging. Semin. Immunol. 69, 101816 (2023).
Zhang, H., Zhang, F. & Modrak, S. Effects of Tnf-Α deletion on inkt cell development, activation, and maturation in the steady-state and chronic alcohol-consuming mice. J. Leukoc. Biol. 112, 233–241 (2022).
Kim, I. H., Kisseleva, T. & Brenner, D. A. Aging and liver disease. Curr. Opin. Gastroenterol. 31, 184–191 (2015).
Hoare, M., Das, T. & Alexander, G. Ageing, telomeres, senescence, and liver injury. J. Hepatol. 53, 950–961 (2010).
Morsiani, C. et al. The peculiar aging of human liver: a geroscience perspective within transplant context. Ageing Res. Rev. 51, 24–34 (2019).
Bailey, K. L. et al. Aging leads to dysfunctional innate immune responses to Tlr2 and Tlr4 agonists. Aging Clin. Exp. Res. 31, 1185–1193 (2019).
Bruunsgaard, H., Skinhøj, P., Pedersen, A. N., Schroll, M. & Pedersen, B. K. Ageing, tumour necrosis factor-alpha (Tnf-Alpha) and atherosclerosis. Clin. Exp. Immunol. 121, 255–260 (2000).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466.e454 (2017).
Kanuri, G., Spruss, A., Wagnerberger, S., Bischoff, S. C. & Bergheim, I. Role of tumor necrosis factor Α (tnfα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 22, 527–534 (2011).
Yin, M. et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117, 942–952 (1999).
Kandhaya-Pillai, R. et al. Tnf-Α/Ifn-Γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated Jak/Stat1. Aging Cell 21, e13646 (2022).
Popa, C., Netea, M. G., van Riel, P. L., van der Meer, J. W. & Stalenhoef, A. F. The role of tnf-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 48, 751–762 (2007).
Tyciakova, S., Valova, V., Svitkova, B. & Matuskova, M. Overexpression of Tnfα induces senescence, autophagy and mitochondrial dysfunctions in melanoma cells. BMC Cancer 21, 507 (2021).
Tomita, K. et al. Tumour necrosis factor alpha signalling through activation of kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 55, 415–424 (2006).
Hern, I. et al. Collagen Α1 (I) gene contains an element responsive to tumor necrosis factor-α located in the 5’untranslated region of its first exon. DNA Cell Biol. 19, 341–352 (2000).
Houglum, K., Buck, M., Kim, D. J. & Chojkier, M. Tnf-alpha inhibits liver collagen-alpha 1(i) gene expression through a tissue-specific regulatory region. Am. J. Physiol. 274, G840–G847 (1998).
Solis-Herruzo, J. A., Brenner, D. A. & Chojkier, M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J. Biol. Chem. 263, 5841–5845 (1988).
Wahid, R. M. et al. Unraveling the hepatic stellate cells mediated mechanisms in aging’s influence on liver fibrosis. Sci. Rep. 14, 13473 (2024).
Shan, L. et al. Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis. Biomed. Pharmacother. 161, 114472 (2023).
Cabral-Pacheco, G. A. et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21249739 (2020).
Gagliano, N. et al. Reduced collagenolytic activity of matrix metalloproteinases and development of liver fibrosis in the aging rat. Mech. Ageing Dev. 123, 413–425 (2002).
Zhang, Y. M. et al. Expression of tissue inhibitor of matrix metalloproteinases-1 during aging in rat liver. World J. Gastroenterol. 11, 3696–3700 (2005).
Delire, B. et al. Aging enhances liver fibrotic response in mice through hampering extracellular matrix remodeling. Aging 9, 98–113 (2016).
Goh, G. B. et al. Age impacts ability of aspartate-alanine aminotransferase ratio to predict advanced fibrosis in nonalcoholic fatty liver disease. Dig. Dis. Sci. 60, 1825–1831 (2015).
Lominadze, Z. & Kallwitz, E. R. Misconception: you can’t have liver disease with normal liver chemistries. Clin. Liver Dis. 12, 96–99 (2018).
Maximos, M. et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology 61, 153–160 (2015).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Kromm, F. et al Aging-related decline in the liver and brain is accelerated by refined diet consumption. Geroscience https://doi.org/10.1007/s11357-025-01897-y (2025).
Wu, Y. L. et al. Gut microbiota alterations and health status in aging adults: from correlation to causation. Aging Med. 4, 206–213 (2021).
Leite, G. et al. Age and the aging process significantly alter the small bowel microbiome. Cell Rep. 36, 109765 (2021).
Conway, J. et al. Age-related loss of intestinal barrier integrity plays an integral role in thymic involution and T cell ageing. Aging Cell 24, e14401 (2025).
Brandt, A. et al. Cognitive alterations in old mice are associated with intestinal barrier dysfunction and induced toll-like receptor 2 and 4 signaling in different brain regions. Cells 12, 2153 (2023).
Vojdani, A. For the assessment of intestinal permeability, size matters. Alter. Ther. Health Med. 19, 12–24 (2013).
Untersmayr, E., Brandt, A., Koidl, L. & Bergheim, I. The intestinal barrier dysfunction as driving factor of inflammaging. Nutrients, 14, https://doi.org/10.3390/nu14050949 (2022).
Drozdowski, L. & Thomson, A. B. Aging and the intestine. World J. Gastroenterol. 12, 7578–7584 (2006).
Choi, J. & Augenlicht, L. H. Intestinal stem cells: guardians of homeostasis in health and aging amid environmental challenges. Exp. Mol. Med. 56, 495–500 (2024).
Wang, F. et al. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166, 409–419 (2005).
Al-Saffar, A. K. et al. Parallel changes in Harvey-Bradshaw index, tnfα, and intestinal fatty acid binding protein in response to infliximab in Crohn’s disease. Gastroenterol. Res. Pr. 2017, 1745918 (2017).
Yakymenko, O. et al. Infliximab restores colonic barrier to adherent-invasive E. coli in Crohn’s disease via effects on epithelial lipid rafts. Scand. J. Gastroenterol. 53, 677–684 (2018).
Mong, P. Y., Petrulio, C., Kaufman, H. L. & Wang, Q. Activation of rho Kinase by Tnf-Α is required for JNK activation in human pulmonary microvascular endothelial cells1. J. Immunol. 180, 550–558 (2008).
Reinhard, C., Shamoon, B., Shyamala, V. & Williams, L. T. Tumor necrosis factor alpha-induced activation of c-jun n-terminal kinase is mediated by Traf2. EMBO J. 16, 1080–1092 (1997).
Sabio, G. & Davis, R. J. Tnf and map kinase signalling pathways. Semin Immunol. 26, 237–245 (2014).
Bu, C. et al. Cell-permeable jnk-inhibitory peptide regulates intestinal barrier function and inflammation to ameliorate necrotizing enterocolitis. J. Cell Mol. Med. 28, e18534 (2024).
Twumasi-Boateng, K. et al. An age-dependent reversal in the protective capacities of JNK signaling shortens Caenorhabditis elegans lifespan. Aging Cell 11, 659–667 (2012).
Chen, J. et al. Age-related changes of microbiota in midlife associated with reduced saccharolytic potential: an in vitro study. BMC Microbiol. 21, 47 (2021).
Pittayanon, R. et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology 158, 930–946.e931 (2020).
Ishaq, M. et al. Microbiota Targeted interventions of probiotic Lactobacillus as an anti-ageing approach: a review. Antioxidants, 10, https://doi.org/10.3390/antiox10121930 (2021).
Lin, Q. et al. The intestinal microbiota modulates the transcriptional landscape of inkt cells at steady-state and following antigen exposure. Mucosal Immunol. 17, 226–237 (2024).
Mandić, A. D. et al. C-jun N-terminal kinase 2 promotes enterocyte survival and goblet cell differentiation in the inflamed intestine. Mucosal Immunol. 10, 1211–1223 (2017).
Zhou, J. & Boutros, M. Jnk-dependent intestinal barrier failure disrupts host-microbe homeostasis during tumorigenesis. Proc. Natl. Acad. Sci. USA 117, 9401–9412 (2020).
Martínez de Toda, I. et al. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech. Ageing Dev. 211, 111797 (2023).
Rajcic, D. et al. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biol. 41, 101879 (2021).
Hamilton, K. L. & Butt, A. G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 37, 415–426 (2013).
Eberts, T. J., Sample, R. H., Glick, M. R. & Ellis, G. H. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin. Chem. 25, 1440–1443 (1979).
Spruss, A., Kanuri, G., Stahl, C., Bischoff, S. C. & Bergheim, I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Investig. 92, 1020–1032 (2012).
Sánchez, V. et al. Oral supplementation of phosphatidylcholine attenuates the onset of a diet-induced metabolic dysfunction-associated steatohepatitis in female C57bl/6j mice. Cell Mol. Gastroenterol. Hepatol. 17, 785–800 (2024).
Jannone, G., Rozzi, M., Najimi, M., Decottignies, A. & Sokal, E. M. An optimized protocol for histochemical detection of senescence-associated beta-galactosidase activity in cryopreserved liver tissue. J. Histochem. Cytochem. 68, 269–278 (2020).
López-De León, A. & Rojkind, M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J. Histochem. Cytochem. 33, 737–743 (1985).
Rao, X., Huang, X., Zhou, Z. & Lin, X. An improvement of the 2ˆ(-Delta Delta Ct) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 3, 71–85 (2013).
Kaewtapee, C. et al. Effect of Bacillus subtilis and Bacillus licheniformis supplementation in diets with low- and high-protein content on ileal crude protein and amino acid digestibility and intestinal microbiota composition of growing pigs. J. Anim. Sci. Biotechnol. 8, 37 (2017).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using qiime 2. Nat. Biotechnol. 37, 852–857 (2019).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Callahan, B. J. et al. Dada2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. Vsearch: a versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. J Mach Learn Res. 12, 2825–2830 (2011).
Quast, C. et al. The silva ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Robeson, M. S. et al. Rescript: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 17, e1009581 (2021).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Faith Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 (1992).
Martino, C. et al. A novel sparse compositional technique reveals microbial perturbations. mSystems, 4, https://doi.org/10.1128/mSystems.00016-19 (2019).
St»hle & Wold Analysis of variance (Anova). Chemom. Intell. Lab. Syst. 6, 259–272 (1989).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 (2001).
Lin, H. & Peddada, S. D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 11, 3514 (2020).
Acknowledgements
This research was funded by the Herzfelder Family Foundation/ Austrian Science Fund FWF (10.55776/P35271 to IB) and the Austrian Science Fund FWF (10.55776/I4844 to IB) and in parts by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 859890 (Smart-Age). For open access purposes, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. We acknowledge the support of the HighPerformance and Cloud Computing Group at the Zentrum für Datenverarbeitung of the University of Tübingen, the state of Baden-Württemberg through bwHPC, and the GermanResearch Foundation (DFG) through grant no. INST 37/935-1FUGG. Graphics in Figure 3 and Graphical Abstract created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Haktan Övül Bozkir: Formal analysis, investigation. Annette Brandt: Formal analysis, Investigation, writing—original draft, writing—review and editing, visualization. Katja Csarmann: Investigation. Anja Baumann: Investigation. Katharina Burger: Investigation, Timur Yergaliyev: Formal analysis, visualization. Tim Hendrikx: Investigation. Amélia Camarinha-Silva: Formal analysis, visualization. Ina Bergheim: Conceptualization, writing—original draft, writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bozkir, H.Ö., Brandt, A., Csarmann, K. et al. TNFα is a trigger of aging-associated liver inflammation in mice. npj Aging (2026). https://doi.org/10.1038/s41514-025-00326-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-025-00326-w