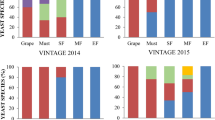
Abstract
Microbial communities play a central role in viticulture, influencing wine characteristics (a concept termed microbial terroir). Yet, the individual factors shaping these microbiomes remain poorly understood. We conducted a multi-year, large-scale survey of Swiss vineyards (95 sites, 680 samples), longitudinally sampling 12 sites (within 2.46 km and identical cultivar and rootstock) over three years. Using 16S rRNA gene and internal transcribed spacer (ITS) amplicon sequencing, untargeted metabolomics (GC-MS, LC-MS/MS), environmental monitoring, and sensory data, we disentangled environmental factors associated with community assembly and fermentation dynamics. Topography and climate collectively structured microbiomes but affected soil- and plant-associated communities differently. Berry-associated fungi showed the strongest site-specific signature, enabling machine-learning predictions of microclimatic variation. Climatic factors and berry chemistry selectively favor fermentative yeasts, which are each linked to distinct metabolite and aroma profiles. Plant stress metabolites were further associated with microbial and metabolite composition. Our integrative approach thereby fundamentally advances our understanding of microbial biogeography and terroir in viticulture.

Similar content being viewed by others
Data availability
Amplicon sequencing data is available from the Sequence Read Archive (SRA) under accession number PRJEB89111 (16S) and PRJEB89112 (ITS).
Code availability
All code used in this study is available on GitHub (https://doi.org/10.5281/zenodo.17530573).
References
Griggs, R. G., et al. Sources and assembly of microbial communities in vineyards as a functional component of winegrowing. Front. Microbiol. 12, https://doi.org/10.3389/fmicb.2021.673810 (2021).
Griggs, R. G. et al. A tale of two vineyards: parsing site-specific differences in bacterial and fungal communities of wine grapes from proximal vineyards, and their changes during processing in a single winery. Appl. Environ. Microbiol. 91, e0052625 (2025). 2025.03.05.641676.
Bokulich, N. A. et al. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. 111, E139–E148 (2014).
Bokulich, N. A., et al. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. mBio 7, https://doi.org/10.1128/mbio.00631-16 (2016).
Papadopoulou, E. et al. Different factors are operative in shaping the epiphytic grapevine microbiome across different geographical scales: biogeography, cultivar or vintage?. J. Sustain. Agric. Environ. 1, 287–301 (2022).
Awad, M. et al. Comparative analysis of grapevine epiphytic microbiomes among different varieties, tissues, and developmental stages in the same terroir. Appl. Sci. 13, 102 (2023).
Flörl, L. et al. The genetic basis of microbiome recruitment in grapevine and its association with fermentative and pathogenic taxa. New Phytol. 248, 178–192 (2025).
Marasco, R. et al. Grapevine rootstocks shape underground bacterial microbiome and networking but not potential functionality. Microbiome 6, 3 (2018).
Burns, K. N. et al. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: differentiation by vineyard management. Soil Biol. Biochem 103, 337–348 (2016).
Coller, E. et al. Microbiome of vineyard soils is shaped by geography and management. Microbiome 7, 140 (2019).
Corneo, P. E., et al. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiol. Ecol. 15 (2013).
Zarraonaindia, I. et al. The soil microbiome influences grapevine-associated microbiota. mBio 6, e02527–14 (2015).
Castañeda, L. E. & Barbosa, O. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in Chilean vineyards and surrounding native forests. PeerJ 5, e3098 (2017).
Knight, S., Klaere, S. & Fedrizzi, B. Regional microbial signatures positively correlate with differential wine phenotypes: evidence for a microbial aspect to terroir. Sci. Rep. 5, 14233 (2015).
Rienth, M. et al. A vine physiology-based terroir study in the AOC-Lavaux region in Switzerland. OENO One 18 (2020).
Adamov, A. ritme. https://doi.org/10.5281/zenodo.14149081 (2024).
Walsh, C. & Vanderburgh, C. Grant, Lady et al. Microbial terroir: associations between soil microbiomes and the flavor chemistry of mustard (Brassica juncea). N. Phytol. 243, 1951–1965 (2024).
Irlinger, F. et al. A comprehensive, large-scale analysis of “terroir” cheese and milk microbiota reveals profiles strongly shaped by both geographical and human factors. ISME Commun. 4, https://doi.org/10.1093/ismeco/ycae095 (2024).
Martín-Miguélez, J. M. et al. Microbial diversity in dry-cured Iberian ham: an approach to the concept of microbial terroir. Int. J. Gastron. Food Sci. 36, 100911 (2024).
Miura, T. et al. Is microbial terroir related to geographic distance between vineyards? Environ. Microbiol. Rep. 9, 742–749 (2017).
Kamilari, E. et al. Metataxonomic analysis of grape microbiota during wine fermentation reveals the distinction of Cyprus regional terroirs. Front. Microbiol. 12, 726483 (2021).
Snyder, T., Osborne, J. & Curtin, C. Contributions of Hanseniaspora species to Pinot Noir microbial terroir in Oregon’s Willamette Valley wine region. Appl. Environ. Microbiol. 0, e00810–e00824 (2024).
Trivedi, P. et al. Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621 (2020).
Martins, G. et al. Characterization of epiphytic bacterial communities from grapes, leaves, bark and soil of grapevine plants grown, and their relations. Abdo Z. (ed.). PLoS ONE 8:e73013 (2013).
Morrison-Whittle, P. & Goddard, M. R. Quantifying the relative roles of selective and neutral processes in defining eukaryotic microbial communities. ISME J. 9, 2003–2011 (2015).
Cao, H. et al. Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci. Rep. 6, 25815 (2016).
Arrigoni, E. et al. Tissue age, orchard location and disease management influence the composition of fungal and bacterial communities present on the bark of apple trees. Environ. Microbiol. 22, 2080–2093 (2020).
Barata, A., Malfeito-Ferreira, M. & Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 153, 243–259 (2012).
Lleixà, J., et al. Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 7, https://doi.org/10.3389/fmicb.2016.00338 (2016).
Hu, K. et al. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 108, 119–127 (2018).
Martin, V. et al. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—a review. Fermentation 4, 76 (2018).
Agarbati, A. et al. The impact of fungicide treatments on yeast biota of Verdicchio and Montepulciano grape varieties. Gerós H. (ed.). PLOS ONE 14:e0217385 (2019).
Hall, M. E. et al. The epiphytic microbiota of sour rot-affected grapes differs minimally from that of healthy grapes, indicating causal organisms are already present on healthy berries. Kurtural S. K. (ed.). PLOS ONE 14:e0211378 (2019).
Tian, B. et al. Extending the influence of terroir through the spontaneous fermentation of Pinot Noir in the vineyard: a case study of greystone vineyard fermentation. J. Agric. Food Chem. 73, 8531–8542 (2025).
Laureano, G. et al. Fatty acid desaturases: uncovering their involvement in grapevine defence against downy mildew. Int. J. Mol. Sci. 22, 5473 (2021).
Goufo, P. & Cortez, I. A lipidomic analysis of leaves of Esca-affected grapevine suggests a role for galactolipids in the defense response and appearance of foliar symptoms. Biology 9, 268 (2020).
Liu, M. et al. Carposphere microbiota alters grape volatiles and shapes the wine grape typicality. N. Phytol. 246, 2280–2294 (2025).
Lazazzara, V., et al. Biogenic volatile organic compounds in the grapevine response to pathogens, beneficial microorganisms, resistance inducers, and abiotic factors. Rosenkranz M. (ed.). J. Exp. Bot. 73:529–554 (2022).
Flörl, L., Meyer, A. & Bokulich, N. A. Exploring sub-species variation in food microbiomes: a roadmap to reveal hidden diversity and functional potential. Appl. Environ. Microbiol. 91, e00524–e00525 (2025).
Estaki M. et al. QIIME 2 enables comprehensive end-to-end analysis of diverse microbiome data and comparative studies with publicly available data. Curr. Protoc. Bioinforma 70, https://doi.org/10.1002/cpbi.100 (2020).
Louw, N. L. et al. Microbiome assembly in fermented foods. Annu. Rev. Microbiol. 77, 381–402 (2023).
Lorenz, D. H. et al. Growth stages of the grapevine: phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1, 100–103 (1995).
Hunter, JJ., Visser, JH. & De Villiers, OT. Preparation of Grapes and Extraction of Sugars and Organic Acids for Determination by High Performance Liquid Chromatography. Am. J. Enol. Vitic. 42, 237–244 (1991).
Mendes Ferreira, A. & Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 9, 1231 (2020).
FOA. Standard operating procedure for soil pH determination (2021).
Flörl, L. et al. HighALPS: Ultra-High-Throughput Marker-Gene Amplicon Library Preparation and Sequencing on the Illumina NextSeq and NovaSeq Platforms. 2024, https://doi.org/10.1101/2024.10.10.617643.
Parada, A. E., Needham, D. M. & Fuhrman, J. A. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 18, 1403–1414 (2016).
Apprill, A. et al. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Micro Ecol. 75, 129–137 (2015).
Bokulich, NA. & Mills, DA. Improved Selection of Internal Transcribed Spacer-Specific Primers Enables Quantitative, Ultra-High-Throughput Profiling of Fungal Communities. Appl. Environ. Microbiol. 79, 2519–2526 (2013).
Lundberg, D. S. et al. Practical innovations for high-throughput amplicon sequencing. Nat. Methods 10, 999–1002 (2013).
Flörl, L. & Bokulich, N. A. Is the juice worth the squeeze? Evaluating Peptide Nucleic Acid (PNA) blockers for reducing plant DNA contamination in 16S rRNA gene sequencing. PhytoFrontiers 5, 17–21 (2024).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10 (2011).
Callahan, B. J. et al. Bioconductor Workflow for Microbiome Data Analysis: from raw reads to community analyses. F1000Research 5, 1492 (2016).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 90 (2018).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41, D590–D596 (2012).
Robeson, M. et al. RESCRIPt: Reproducible sequence taxonomy reference database management. PLOS Comput. Biol. 17, e1009581 (2021).
Kaehler, B. D. et al. Species abundance information improves sequence taxonomy classification accuracy. Nat. Commun. 10, 4643 (2019).
Abarenkov, K. et al. UNITE QIIME release for Fungi. https://doi.org/10.15156/BIO/2938079 (2023).
Davis, N. M., Proctor, D. M., Holmes, S. P., Relman, D. A. & Callahan, B. J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 226 (2018).
Pielou, E. C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144 (1966).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Technol. J. 27, 379–423 (1948).
Sorensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. 5, 1–34 (1948).
Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44, 223–270 (1908).
Bokulich, N. A. Integrating sequence composition information into microbial diversity analyses with k-mer frequency counting. mSystems e01550-24 (2025).
Price, M. N., Dehal, P. S., Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. Poon A. F. Y. (ed.). PLoS ONE 5:e9490 (2010).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Lozupone, C. et al. UniFrac: an effective distance metric for microbial community comparison. ISME J. 5, 169–172 (2011).
Kruskal, W. H. & Wallis, W. A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621 (1952).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 (2001).
Oksanen, J. et al. vegan: Community Ecol. Package 2, 6–8 (2001).
Bokulich, N. et al. q2-sample-classifier: machine-learning tools for microbiome classification and regression. J. Open Source Softw. 3, 934 (2018).
Halford, M. Prince. https://github.com/MaxHalford/prince (2025).
Rohart, F. et al. mixOmics: an R package for ‘omics feature selection and multiple data integration. Schneidman D. (ed.). PLOS Comput. Biol. 2017;13:e1005752.
Foster, Z. S. L., Sharpton, T. J., Grünwald, N. J. Metacoder: an R package for visualization and manipulation of community taxonomic diversity data. Poisot T. (ed.). PLOS Comput. Biol. 13:e1005404 (2017).
Lin, H. & Peddada, S. D. Multigroup analysis of compositions of microbiomes with covariate adjustments and repeated measures. Nat. Methods 21, 83–91 (2024).
The Matplotlib Development Team. Matplotlib: Visualization with Python. https://doi.org/10.5281/ZENODO.13308876 (2024).
Waskom, M. seaborn: statistical data visualization. J. Open Source Softw. 6, 3021 (2021).
Jordahl, K., et al. geopandas/geopandas: v0.8.1. 2020, https://doi.org/10.5281/ZENODO.3946761.
Noonan, M. J., Tinnesand, H. V. & Buesching, C. D. Normalizing Gas-Chromatography–Mass Spectrometry Data: Method Choice can Alter Biological Inference. BioEssays 40, 1700210 (2018).
Wiley. Wiley Registry of Mass Spectral Data, 10th Edition.
National Institute of Standards and Technology. NIST Mass Spectral Library (Version).
Mondello, Luigi. Wiley FFNSC Library - Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds, 3rd Edition. 2015.
HighChem LLC. mzCloud - Advanced Mass Spectral Database.
Thermo Fisher Scientific Inc. mzVault.
Royal Society of Chemistry. ChemSpider. 2025.
Bioinformatics Research Group, SRI International. BioCyc.
Wishart, D. S. et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 35, D521–D526 (2007).
Kanehisa, M. et al. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Neveu, V. et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database 2010, bap024 (2010).
Hawkins, C. et al. Plant Metabolic Network 16: expansion of underrepresented plant groups and experimentally supported enzyme data. Nucleic Acids Res. 53, D1606–D1613 (2025).
Arita Group. Arita Lab 6549 flavonoid structure database. http://metabolomics.jp/wiki/Main_Page.
Poynton, E. F. et al. The Natural Products Atlas 3.0: extending the database of microbially derived natural products. Nucleic Acids Res. 53, D691–D699 (2025).
Thermo Fisher Scientific Inc. mzLogic Data Analysis Algorithm: Accelerate small-molecule unknown identification. thermofisher.com/mzLogic (2019).
Acknowledgements
We would like to thank the winegrowers of the Villette AOC Lavaux and Valais for letting us collect samples over 3 years. Further we thank Vivian Zufferey and Jean-Sébastien Reynard of Agroscope for collecting samples in Valais, Serafina Plüss and Julinka Wäsche for technical support, Ondino Azario and Laura Nyström (ETH Zürich) for supporting the GC-MS analysis, and Alfonso Die for supporting HPLC analysis. The authors thank the Genetic Diversity Centre (GDC) of ETH Zurich for supporting the library preparation. The microbiome amplicon sequencing and LC-MS metabolomics analysis were performed at the Functional Genomics Center Zurich (FGCZ) of University of Zurich and ETH Zurich. The authors gratefully acknowledge financial support from the Swiss National Science Foundation [Grant Number: 310030_204275] (to N.A.B.) and the Swiss Government Excellence Ph.D. Scholarship (to L.F.).
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich.
Author information
Authors and Affiliations
Contributions
The study was conceived by N.A.B. and M.R., and supervised by N.A.B. L.F. and P.S. collected samples, and P.S. recorded viticultural metrics and performed the microvinifications. L.F. processed the samples, performed the analysis, and wrote the article with review and contributions from N.A.B., M.R. and P.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flörl, L., Schönenberger, P., Rienth, M. et al. Grape expectations: disentangling environmental drivers of microbiome establishment in winegrowing ecosystems. npj Biofilms Microbiomes (2026). https://doi.org/10.1038/s41522-026-00915-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-026-00915-x