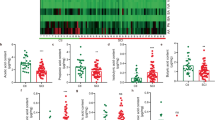
Abstract
Cognitive impairment (CI) following mild traumatic brain injury (mTBI) poses a clinical challenge, with emerging evidence implicating gut microbiota. This study found that mTBI patients who developed CI exhibited decreased Hungatella hathewayi, while those without CI showed an increase. Microbiota transplantation in mTBI rats revealed that higher Hungatella hathewayi levels enriched beneficial, short-chain fatty acid (SCFA) -producing bacteria and reduced harmful ones. Elevated Hungatella hathewayi improved performance in the Morris water maze and novel object recognition tests, indicating enhanced spatial learning and memory. It also reduced gut and brain inflammation, shown by lower TNF-α and IL-6 mRNA expression, and promoted M2 microglia polarization in the peri-lesional cortex. Metabolomics identified increased fecal and serum butyrate, a SCFA with anti-neuroinflammatory properties. Thus, Hungatella hathewayi may mitigate Post-mTBI CI by boosting butyrate production, which alleviates intestinal inflammation, shifts microglia toward the protective M2 phenotype, reduces neuroinflammation, and supports neuroprotection, ultimately lowering CI risk after mTBI. This study was registered with the Chinese Clinical Trial Registry (ChiCTR) on May 31, 2023 (Registration number: ChiCTR2300072000, URL: https://www.chictr.org.cn/showproj.html?proj=197867).
Similar content being viewed by others
Data availability
The data supporting this study cannot be made publicly available due to ethicaland privacy concerns, as they contain sensitive information that could compromise the confidentiality of human subjects. Access to the data is restricted to protect participant anonymity and comply with institutional and legal requirements. Requests for limited, anonymized data may be considered on a case-by-case basis, subject to approval by the relevant ethics committee.
Code availability
The underlying code for this study is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author.
References
Dewan, M. C. et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130, 1080–1097 (2018).
McInnes, K., Friesen, C. L., MacKenzie, D. E., Westwood, D. A. & Boe, S. G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One 12, e0174847 (2017).
Stika, M. M. et al. Cognition and other predictors of functional disability among veterans with mild traumatic brain injury and posttraumatic stress disorder. J. Head. Trauma Rehabil. 36, 44–55 (2021).
Wang, S. et al. Temporal changes of the oral and fecal microbiota after mild traumatic brain injury in rats by 16S rRNA sequencing. Microorganisms 11, 1452 (2023).
Aghakhani, N. Relationship between mild traumatic brain injury and the gut microbiome: A scoping review. J. Neurosci. Res. 100, 827–834 (2022).
Rice, M. W., Pandya, J. D. & Shear, D. A. Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries. Front Neurol. 10, 875 (2019).
D’Amato, A. et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 8, 140 (2020).
Han, L. et al. Palmatine improves cognitive dysfunction in Alzheimer’s disease model rats through autophagy pathway and regulation of gut microbiota. Brain Res. 1835, 148932 (2024).
Liu, N., Yang, D., Sun, J. & Li, Y. Probiotic supplements are effective in people with cognitive impairment: a meta-analysis of randomized controlled trials. Nutr. Rev. 81, 1091–1104 (2023).
Amgalan, A. et al. Functional connectome dynamics after mild traumatic brain injury according to age and sex. Front. Aging Neurosci. 14, 852990 (2022).
Hallock, H. et al. Sport-related concussion: a cognitive perspective. Neurol. Clin. Pr. 13, e200123 (2023).
Yang, J. et al. The gut microbiota modulates neuroinflammation in Alzheimer’s disease: elucidating crucial factors and mechanistic underpinnings. CNS Neurosci. Ther. 30, e70091 (2024).
Trichka, J. & Zou, W.-Q. Modulation of neuroinflammation by the gut microbiota in Prion and Prion-$eases. Pathogens 10, 887 (2021).
Ullah, H. et al. The gut microbiota-brain axis in neurological disorder. Front. Neurosci. 17, 1225875 (2023).
Misiak, B. et al. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 102, 109951 (2020).
Houlden, A. et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 57, 10–20 (2016).
Ge, X. et al. Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J. Clin. Invest. 133, e154612 (2023).
Solanki, R., Karande, A. & Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front Neurol. 14, 1149618 (2023).
Wang, X., Wang, Z., Cao, J., Dong, Y. & Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 11, 17 (2023).
Fu, X., Liu, Z., Zhu, C., Mou, H. & Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 59, S130–S152 (2019).
Ezzat-Zadeh, Z. et al. California strawberry consumption increased the abundance of gut microorganisms related to lean body weight, health and longevity in healthy subjects. Nutr. Res. 85, 60–70 (2021).
Singh, V. et al. Butyrate producers, ‘The Sentinel of Gut’: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 13, 1103836 (2023).
Yuan, X. et al. Depression and anxiety in patients with active ulcerative colitis: crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes. 13, 1987779 (2021).
Sun, J., Li, J., Cheng, G., Sha, B. & Zhou, W. Effects of hypothermia on NSE and S-100 protein levels in CSF in neonates following hypoxic/ischaemic brain damage. Acta Paediatr. 101, e316–e320 (2012).
Wang, H. et al. Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J. Neuroinflamm. 19, 76 (2022).
Sun, Y. et al. Elevated Serum Levels Of Inflammation-related Cytokines In Mild Traumatic Brain Injury Are Associated With Cognitive Performance. Front. Neurol. 10, 1120 (2019).
Wagner, C. et al. Differentiation paths of Peyer’s Patch LysoDCs are linked to sampling site positioning, migration, and T cell priming. Cell Rep. 31, 107479 (2020).
Altomare, A. et al. Gut mucosal-associated microbiota better discloses inflammatory bowel disease differential patterns than faecal microbiota. Dig. Liver Dis. 51, 648–656 (2019).
Karaca, O. & Doğan, G. The effects of dexmedetomidine in increased intestinal permeability after traumatic brain injury: An experimental study. Ulus. Travma Acids Cerrahi Derg. 26, 15–20 (2020).
Fink, L. N. et al. Establishment of tolerance to commensal bacteria requires a complex microbiota and is accompanied by decreased intestinal chemokine expression. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G55–G65 (2012).
Thaiss, C. A. et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359, 1376–1383 (2018).
Tang, T., Wang, X., Qi, E., Li, S. & Sun, H. Ginkgetin promotes M2 polarization of Microglia and exert neuroprotection in ischemic stroke via modulation of PPARγ pathway. Neurochem Res 47, 2963–2974 (2022).
Sawoo, R., Dey, R., Ghosh, R. & Bishayi, B. Exogenous IL-10 posttreatment along with TLR4 and TNFR1 blockade improves tissue antioxidant status by modulating sepsis-induced macrophage polarization. J. Appl Toxicol. 43, 1549–1572 (2023).
Francos-Quijorna, I., Amo-Aparicio, J., Martinez-Muriana, A. & López-Vales, R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 64, 2079–2092 (2016).
Zhou, C., Sun, P., Hamblin, M. H. & Yin, K.-J. Genetic deletion of Krüppel-like factor 11 aggravates traumatic brain injury. J. Neuroinflamm. 19, 281 (2022).
Felice, C., Lewis, A., Armuzzi, A., Lindsay, J. O. & Silver, A. Review article: selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment Pharm. Ther. 41, 26–38 (2015).
Fragas, M. G., Oliveira, D. M. de, Hiyane, M. I., Braga, T. T. & Camara, N. O. S. The dual effect of acetate on microglial TNF-α production. Clinics 77, 100062 (2022).
Jin, M.-Y. et al. Noni (Morinda citrifolia L.) Fruit Polysaccharides Regulated IBD Mice Via Targeting Gut Microbiota: Association of JNK/ERK/NF-κB Signaling Pathways. J. Agric Food Chem. 69, 10151–10162 (2021).
Proia, P., Di Liegro, C. M., Schiera, G., Fricano, A. & Di Liegro, I. Lactate as a metabolite and a regulator in the central nervous system. Int J. Mol. Sci. 17, 1450 (2016).
Jorwal, P. & Sikdar, S. K. Lactate reduces epileptiform activity through HCA1 and GIRK channel activation in rat subicular neurons in an in vitro model. Epilepsia 60, 2370–2385 (2019).
Guo, Y., Cho, S. W., Saxena, D. & Li, X. Multifaceted actions of succinate as a signaling transmitter vary with its cellular locations. Endocrinol. Metab. 35, 36–43 (2020).
Ortiz-Masiá, D. et al. Succinate activates EMT in intestinal epithelial cells through SUCNR1: a novel protagonist in fistula development. Cells 9, 1104 (2020).
Yang, W., Ren, D., Zhao, Y., Liu, L. & Yang, X. Fuzhuan brick tea polysaccharide improved ulcerative colitis in association with gut microbiota-derived Tryptophan metabolism. J. Agric Food Chem. 69, 8448–8459 (2021).
Sun, J. et al. Microbiota-derived metabolite Indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav. Immun. 106, 76–88 (2022).
Baars, A., Oosting, A., Knol, J., Garssen, J. & van Bergenhenegouwen, J. The gut microbiota as a therapeutic target in IBD and metabolic disease: a role for the bile acid receptors FXR and TGR5. Microorganisms 3, 641–666 (2015).
Ramírez-Pérez, O., Cruz-Ramón, V., Chinchilla-López, P. & Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 16, s15–s20 (2017).
Committee AMTBI Definition of mild traumatic brain injury. J. Head. Trauma Rehabil. 8, 86–87 (1993).
Gil-Berrozpe, G. J. et al. Utility of the MoCA for cognitive impairment screening in long-term psychosis patients. Schizophr. Res. 216, 429–434 (2020).
Lin, Z. et al. Reliability and validity of the Chinese version of the Montreal Cognitive Assessment in patients with craniocerebral injury. J. Qiqihar. Med. Univ. 33, 1424–1426 (2012).
Arevalo-Rodriguez, I. et al. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 7, CD010783 (2021).
Zhou X. Preliminary study on the reliability and validity of the Chinese version of the Mini-Mental State Examination in stroke patients. Fujian University of Traditional Chinese Medicine (2015).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Russell, J. K., Jones, C. K. & Newhouse, P. A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 16, 649–665 (2019).
Feeney, D. M., Boyeson, M. G., Linn, R. T., Murray, H. M. & Dail, W. G. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 211, 67–77 (1981).
Hou, J. et al. Mild and Mild-to-Moderate Traumatic Brain Injury-Induced Significant Progressive and Enduring Multiple Comorbidities. J. Neurotrauma 34, 2456–2466 (2017).
Braeckman, K., Descamps, B., Vanhove, C. & Caeyenberghs, K. Exploratory relationships between cognitive improvements and training induced plasticity in hippocampus and cingulum in a rat model of mild traumatic brain injury: a diffusion MRI study. Brain Imaging Behav. 14, 2281–2294 (2020).
Su, S.-H. et al. Fecal microbiota transplantation and short-chain fatty acids protected against cognitive dysfunction in a rat model of chronic cerebral hypoperfusion. CNS Neurosci. Ther. 29, 98–114 (2023).
Damodaran, T. et al. Clitoria ternatea L. root extract ameliorated the cognitive and hippocampal long-term potentiation deficits induced by chronic cerebral hypoperfusion in the rat. J. Ethnopharmacol. 224, 381–390 (2018).
Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process 13, 93–110 (2012).
Wu, H. et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J. Neuroinflamm. 18, 2 (2021).
Zou, Z. et al. Naturally-occurring spinosyn A and its derivatives function as argininosuccinate synthase activator and tumor inhibitor. Nat. Commun. 12, 2263 (2021).
Nossa, C. W. et al. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 16, 4135–4144 (2010).
Wu, L. et al. Phasing amplicon sequencing on Illumina Miseq for robust environmental microbial community analysis. BMC Microbiol. 15, 125 (2015).
Li, D., Liu, C.-M., Luo, R., Sadakane, K. & Lam, T.-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Li, D. et al. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 102, 3–11 (2016).
Acknowledgements
We would like to acknowledge the National Natural Science Foundation of China and the Sichuan Provincial Department of Science and Technology for their financial support of this research. (Grant number: U22A20334, 2024NSFSC0592, and 2025ZNSFSC1691).
Author information
Authors and Affiliations
Contributions
D.Q., L.Q., and L.K. conducted the entire experiment and wrote the manuscript; L.Q. and L.K. are responsible for the paper; W.Y., L.G., X.X., and Y.J. prepared Figs. 1–7 and Tables 1–3; H.U. polished the language of the paper. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, Q., Li, Q., Ullah, H. et al. Harnessing gut microbiota for brain health: protective role of Hungatella hathewayi for post-mTBI cognitive impairment. npj Biofilms Microbiomes (2026). https://doi.org/10.1038/s41522-026-00922-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-026-00922-y